Ullmann condensation
| Ullmann condensation | |
|---|---|
| Named after | Fritz Ullmann |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | ullmann-reaction |
| RSC ontology ID | RXNO:0000081 |
In the Ullmann condensation or Ullmann-type reaction is the copper-promoted conversion of aryl halides to aryl ethers, aryl thioethers, aryl nitriles, and aryl amines. These reactions are examples of cross-coupling reactions.[1]
Ullmann-type reactions are comparable to Buchwald–Hartwig reactions but usually require higher temperatures. Traditionally these reaction requires high-boiling polar solvents such as N-methylpyrrolidone, nitrobenzene, or dimethylformamide and high temperatures (often in excess of 210 °C) with stoichiometric amounts of copper. Aryl halide were required to be activated by electron-withdrawing groups. Traditional Ullmann style reactions used "activated" copper powder, e.g. prepared in situ by the reduction of copper sulfate by zinc metal in hot water. The methodology improved with the introduction of soluble copper catalysts supported by diamines and acetylacetonate ligands.[1]
Ullmann ether synthesis: C-O coupling[]
Illustrative of the traditional Ullmann ether synthesis is the preparation of p-nitrophenyl phenyl ether from 4-chloronitrobenzene and phenol.[2]
Modern arylations use soluble copper catalysts.[3]
Goldberg reaction: C-N coupling[]
This section may be confusing or unclear to readers. (May 2020) |
A traditional Goldberg reaction is illustrated by the synthesis of fenamic acid, an intermediate in the preparation of acridone:[4]

Aryl iodides are favored arylating agents.[5] The catalyst used is formed from copper(I) iodide and phenanthroline. As this reaction proceeds well with an electron-rich aryl iodide it is a valuable alternative to the Buchwald–Hartwig amination reaction, which gives best yields with electron-poor aryl halides. The scope is extended to amides.[1]

Hurtley reaction: C-C coupling[]
The nucleophile can also be carbon as in a carbanion as well as cyanide. In the traditional Hurtley reaction, the carbon nucleophiles were derived from malonic ester and other dicarbonyl compounds:[7]
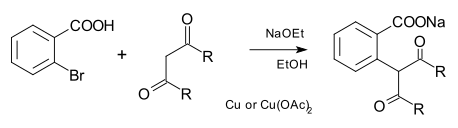
More modern Cu-catalyzed C-C cross-couplings utilize soluble copper complexes containing phenanthroline ligands.[8]
C–S coupling[]
The arylation of alkylthiolates proceeds by the intermediacy of cuprous thiolates.[9]
Mechanism of Ullmann-type reactions[]
In the case of Ullmann-type reactions (aminations, etherifications, etc. of aryl halides), the reaction involves copper(I) alkoxide, copper(I) amides, copper(I) thiolates. The copper(I) reagent can be generated in situ from the aryl halide and copper metal. Even copper(II) sources are effective. A number of innovations have been developed with regards to copper reagents.[1]
These copper(I) compounds subsequently react with the aryl halide in a net metathesis reaction:
- Ar-X + ROCu → Ar-OR + CuX
- Ar-X + RSCu → Ar-SR + CuX
- Ar-X + 2 RNHCu → Ar-NHR + CuX
In the case of C-N coupling, kinetic studies implicate oxidative addition reaction followed by reductive elimination from Cu(III) intermediates (Ln = one or more spectator ligands):[10]
- ROCuAr(X)Ln → RO-Ar + CuLn
History[]
The Ullmann ether synthesis or is named after its inventor, Fritz Ullmann.[11] The corresponding Goldberg reaction, is named after Irma Goldberg.[12] The Hurtley reaction, which involves C-C bond formation, is similarly named after its inventor.[7]
References[]
- ^ Jump up to: a b c d Florian Monnier, Marc Taillefer (2009). "Minireview Catalytic CC, CN, and CO Ullmann-Type Coupling Reactions". Angewandte Chemie International Edition. 48 (38): 6954–71. doi:10.1002/anie.200804497. PMID 19681081.
- ^ Ray Q. Brewster, Theodore Groening (1934). "p-Nitrodiphenyl Ether". Org. Synth. 14: 66. doi:10.15227/orgsyn.014.0066.CS1 maint: uses authors parameter (link)
- ^ Buck, Elizabeth; Song, Zhiguo J. (2005). "Preparation of 1-Methoxy-2-(4-Methoxyphenoxy)Benzene". Organic Syntheses. 82: 69. doi:10.15227/orgsyn.082.0069.
- ^ C. F. H. Allen, G. H. W. McKee (1939). "Acridone". Organic Syntheses. 2: 6. doi:10.15227/orgsyn.019.0006.
- ^ H.B. Goodbrand; Nan-Xing Hu (1999). "Ligand-Accelerated Catalysis of the Ullmann Condensation: Application to Hole Conducting Triarylamines". Journal of Organic Chemistry. 64 (2): 670–674. doi:10.1021/jo981804o.
- ^ Jones, C. P.; Anderson, K. W.; Buchwald, S. L. (2007). "Sequential Cu-Catalyzed Amidation-Base-Mediated Camps Cyclization: A Two-Step Synthesis of 2-Aryl-4-quinolones from o-Halophenones". J. Org. Chem. 72 (21): 7968–7973. doi:10.1021/jo701384n. PMID 17850097.
- ^ Jump up to: a b William Robert Hardy Hurtley (1929). "Replacement of Halogen in ortho-Bromobenzoic Acid". J. Chem. Soc.: 1870. doi:10.1039/JR9290001870.
- ^ Antoine Nitelet, Sara Zahim, Cédric Theunissen, Alexandre Pradal, Gwilherm Evano (2016). "Copper-catalyzed Cyanation of Alkenyl Iodides". Org. Synth. 93: 163. doi:10.15227/orgsyn.093.0163.CS1 maint: multiple names: authors list (link)
- ^ Roger Adams, Walter Reifschneider, Aldo Ferretti (1962). "1,2-Bis(N-butylthio)benzene". Org. Synth. 42: 22. doi:10.15227/orgsyn.042.0022.CS1 maint: multiple names: authors list (link)
- ^ Ramesh Giri, Andrew Brusoe, Konstantin Troshin, Justin Y. Wang, Marc Font, John F. Hartwig (2018). "Mechanism of the Ullmann Biaryl Ether Synthesis Catalyzed by Complexes of Anionic Ligands: Evidence for the Reaction of Iodoarenes with Ligated Anionic CuI Intermediates". J. Am. Chem. Soc. 140 (2): 793–806. doi:10.1021/jacs.7b11853. PMC 5810543. PMID 29224350.CS1 maint: uses authors parameter (link)
- ^ Fritz Ullmann, Paul Sponagel (1905). "Ueber die Phenylirung von Phenolen". Berichte der deutschen chemischen Gesellschaft. 38 (2): 2211–2212. doi:10.1002/cber.190503802176.
- ^ Irma Goldberg (1906). "Ueber Phenylirungen bei Gegenwart von Kupfer als Katalysator". Berichte der deutschen chemischen Gesellschaft. 39 (2): 1691–1692. doi:10.1002/cber.19060390298.
- Coupling reactions
- Carbon-heteroatom bond forming reactions
- Condensation reactions
- Name reactions
