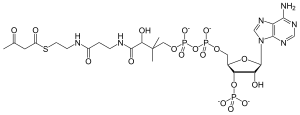
Acetoacetyl-CoA
| |
| Names | |
|---|---|
| Preferred IUPAC name
S-{(9R)-1-[(2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]-3,5,9-trihydroxy-8,8-dimethyl-3,5,10,14-tetraoxo-2,4,6-trioxa-11,15-diaza-3λ5,5λ5-diphosphaheptadecan-17-yl} 3-oxobutanethioate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.378 |
IUPHAR/BPS
|
|
| MeSH | acetoacetyl+CoA |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C25H40N7O18P3S | |
| Molar mass | 851.609 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Acetoacetyl CoA is the precursor of HMG-CoA in the mevalonate pathway, which is essential for cholesterol biosynthesis. It also takes a similar role in the ketone bodies synthesis (ketogenesis) pathway of the liver. In the ketone bodies digestion pathway (in the tissue), it is no longer associated with having HMG-CoA as a product or as a reactant.
It is created from acetyl-CoA, a thioester, which reacts with the enolate of a second molecule of acetyl-CoA in a Claisen condensation reaction,[1] and it is acted upon by HMG-CoA synthase to form HMG-CoA. During the metabolism of leucine, this last reaction is reversed. Some individuals may experience Acetoacetyl-CoA deficiency.[2] This deficiency is classified as a disorder ketone body and isoleucine metabolism that can be inherited.[3]

References[]
- ^ Yurkanis, Bruice, Paula (2017). Organic chemistry. Pearson. ISBN 9780134042282. OCLC 974910578.
- ^ Tsuda, Hirohisa; Shiraki, Mari; Inoue, Eri; Saito, Terumi (August 2016). "Generation of poly-β-hydroxybutyrate from acetate in higher plants: Detection of acetoacetyl CoA reductase- and PHB synthase- activities in rice". Journal of Plant Physiology. 201: 9–16. doi:10.1016/j.jplph.2016.06.007. ISSN 0176-1617. PMID 27372278.
- ^ Bose, K. S.; Sarma, R. H. (1975-10-27). "Delineation of the intimate details of the backbone conformation of pyridine nucleotide coenzymes in aqueous solution". Biochemical and Biophysical Research Communications. 66 (4): 1173–1179. doi:10.1016/0006-291x(75)90482-9. ISSN 1090-2104. PMID 2.
See also:
- Mevalonate pathway
- Acetoacetic acid
- Beta-hydroxybutyryl-CoA dehydrogenase
- Thioesters of coenzyme A
- Biochemistry stubs