Tyrosine
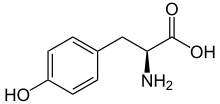 Skeletal formula of L-tyrosine
| |||
 L-Tyrosine at physiological pH
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(S)-Tyrosine
| |||
| Other names
L-2-Amino-3-(4-hydroxyphenyl)propanoic acid
| |||
| Identifiers | |||
CAS Number
|
| ||
3D model (JSmol)
|
| ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.419 | ||
IUPHAR/BPS
|
|||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
show
InChI | |||
show
SMILES | |||
| Properties | |||
Chemical formula
|
C9H11NO3 | ||
| Molar mass | 181.191 g·mol−1 | ||
Solubility in water
|
.0453 g/100 mL | ||
Magnetic susceptibility (χ)
|
-105.3·10−6 cm3/mol | ||
| Hazards | |||
| Safety data sheet | See: data page | ||
| NFPA 704 (fire diamond) | 
1
1
0 | ||
| Supplementary data page | |||
Structure and
properties |
Refractive index (n), Dielectric constant (εr), etc. | ||
Thermodynamic
data |
Phase behaviour solid–liquid–gas | ||
Spectral data
|
UV, IR, NMR, MS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
L-Tyrosine or tyrosine (symbol Tyr or Y)[2] or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek tyrós, meaning cheese, as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese.[3][4] It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine.[5] It is encoded by the codons UAC and UAU in messenger RNA.
Functions[]
Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hydroxyl group can change the activity of the target protein, or may form part of a signaling cascade via SH2 domain binding.
A tyrosine residue also plays an important role in photosynthesis. In chloroplasts (photosystem II), it acts as an electron donor in the reduction of oxidized chlorophyll. In this process, it loses the hydrogen atom of its phenolic OH-group. This radical is subsequently reduced in the photosystem II by the four core manganese clusters.
Dietary requirements and sources[]
The Dietary Reference Intake (recommended dietary allowance, RDA) for phenylalanine and tyrosine is 42 mg per kilogram of body weight.[6] For a 70 kg person, this is 2.31 g (phenylalanine + tyrosine).
Tyrosine, which can also be synthesized in the body from phenylalanine, is found in many high-protein food products such as chicken, turkey, fish, milk, yogurt, cottage cheese, cheese, peanuts, almonds, pumpkin seeds, sesame seeds, soy products and lima beans, but also in avocados and bananas.[7] For example, the white of an egg has about 250 mg per egg,[8] while lean beef/lamb/pork/salmon/chicken/turkey contains about 1 g per 3 ounces (85 g) portion.[8]
Biosynthesis[]

In plants and most microorganisms, tyr is produced via prephenate, an intermediate on the shikimate pathway. Prephenate is oxidatively decarboxylated with retention of the hydroxyl group to give p-hydroxyphenylpyruvate, which is transaminated using glutamate as the nitrogen source to give tyrosine and α-ketoglutarate.
Mammals synthesize tyrosine from the essential amino acid phenylalanine (phe), which is derived from food. The conversion of phe to tyr is catalyzed by the enzyme phenylalanine hydroxylase, a monooxygenase. This enzyme catalyzes the reaction causing the addition of a hydroxyl group to the end of the 6-carbon aromatic ring of phenylalanine, such that it becomes tyrosine.
Metabolism[]

Phosphorylation and sulfation[]
Some of the tyrosine residues can be tagged (at the hydroxyl group) with a phosphate group (phosphorylated) by protein kinases. In its phosphorylated form, tyrosine is called phosphotyrosine. Tyrosine phosphorylation is considered to be one of the key steps in signal transduction and regulation of enzymatic activity. Phosphotyrosine can be detected through specific antibodies. Tyrosine residues may also be modified by the addition of a sulfate group, a process known as tyrosine sulfation.[9] Tyrosine sulfation is catalyzed by tyrosylprotein sulfotransferase (TPST). Like the phosphotyrosine antibodies mentioned above, antibodies have recently been described that specifically detect sulfotyrosine.[10]
Precursor to neurotransmitters and hormones[]
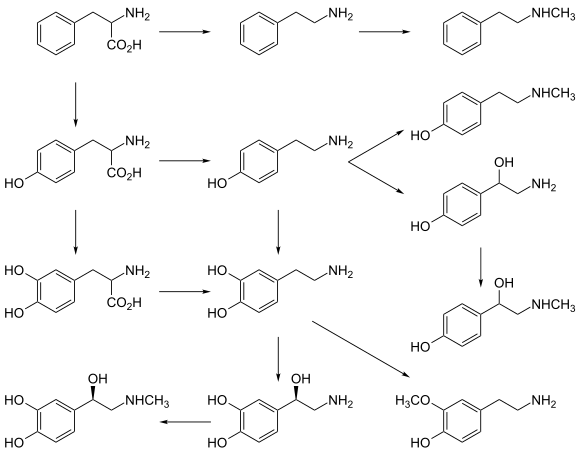
In dopaminergic cells in the brain, tyrosine is converted to L-DOPA by the enzyme tyrosine hydroxylase (TH). TH is the rate-limiting enzyme involved in the synthesis of the neurotransmitter dopamine. Dopamine can then be converted into other catecholamines, such as norepinephrine (noradrenaline) and epinephrine (adrenaline).
The thyroid hormones triiodothyronine (T3) and thyroxine (T4) in the colloid of the thyroid are also derived from tyrosine.
Precursor to alkaloids[]
The latex of Papaver somniferum, the opium poppy, has been shown to convert tyrosine into the alkaloid morphine and the bio-synthetic pathway has been established from tyrosine to morphine by using Carbon-14 radio-labelled tyrosine to trace the in-vivo synthetic route.[citation needed]
Precursor to natural phenols[]
Tyrosine ammonia lyase (TAL) is an enzyme in the natural phenols biosynthesis pathway. It transforms L-tyrosine into p-coumaric acid.
Precursor to pigments[]
Tyrosine is also the precursor to the pigment melanin.
Role in coenzyme Q10 synthesis[]
Tyrosine (or its precursor phenylalanine) is needed to synthesize the benzoquinone structure which forms part of coenzyme Q10.
Degradation[]

The decomposition of L-tyrosine (syn. para-hydroxyphenylalanine) begins with an α-ketoglutarate dependent transamination through the tyrosine transaminase to para-hydroxyphenylpyruvate. The positional description para, abbreviated p, mean that the hydroxyl group and side chain on the phenyl ring are across from each other (see the illustration below).
The next oxidation step catalyzes by p-hydroxyphenylpyruvate dioxygenase and splitting off CO2 homogentisate (2,5-dihydroxyphenyl-1-acetate).[14] In order to split the aromatic ring of homogentisate, a further dioxygenase, homogentisate 1,2-dioxygenase is required. Thereby, through the incorporation of a further O2 molecule, maleylacetoacetate is created.
Fumarylacetoacetate is created by maleylacetoacetate cis-trans-isomerase through rotation of the carboxyl group created from the hydroxyl group via oxidation. This cis-trans-isomerase contains glutathione as a coenzyme. Fumarylacetoacetate is finally split by the enzyme fumarylacetoacetate hydrolase through the addition of a water molecule.
Thereby fumarate (also a metabolite of the citric acid cycle) and acetoacetate (3-ketobutyroate) are liberated. Acetoacetate is a ketone body, which is activated with succinyl-CoA, and thereafter it can be converted into acetyl-CoA, which in turn can be oxidized by the citric acid cycle or be used for fatty acid synthesis.
Phloretic acid is also a urinary metabolite of tyrosine in rats.[15]
Ortho- and meta-tyrosine[]

Three structural isomers of L-tyrosine are known. In addition to the common amino acid L-tyrosine, which is the para isomer (para-tyr, p-tyr or 4-hydroxyphenylalanine), there are two additional regioisomers, namely meta-tyrosine (also known as 3-hydroxyphenylalanine, L-m-tyrosine, and m-tyr) and ortho-tyrosine (o-tyr or 2-hydroxyphenylalanine), that occur in nature. The m-tyr and o-tyr isomers, which are rare, arise through non-enzymatic free-radical hydroxylation of phenylalanine under conditions of oxidative stress.[16][17]
m-Tyrosine and analogues (rare in nature but available synthetically) have shown application in Parkinson's disease, Alzheimer's disease and arthritis.[18]
Medical use[]
Tyrosine is a precursor to neurotransmitters and increases plasma neurotransmitter levels (particularly dopamine and norepinephrine),[19] but has little if any effect on mood in normal subjects.[20][21][22] However, a number of studies have found tyrosine to be useful during conditions of stress, cold, fatigue (in mice),[23] prolonged work and sleep deprivation,[24][25] with reductions in stress hormone levels,[26] reductions in stress-induced weight loss seen in animal trials,[23] and improvements in cognitive and physical performance[21][27][28] seen in human trials.
Tyrosine does not seem to have any significant effect on cognitive or physical performance in normal circumstances,[29][30] but does help sustain working memory better during multitasking.[31]
Industrial synthesis[]
L-tyrosine and its derivatives (L-DOPA, melanin, phenylpropanoids, and others) are used in pharmaceuticals, dietary supplements, and food additives. Two methods were formerly used to manufacture L-tyrosine. The first involves the extraction of the desired amino acid from protein hydrolysates using a chemical approach. The second utilizes enzymatic synthesis from phenolics, pyruvate, and ammonia through the use of tyrosine phenol-lyase.[32] Advances in genetic engineering and the advent of industrial fermentation have shifted the synthesis of L-tyrosine to the use of engineered strains of E. coli.[33][32]
See also[]
- Albinism
- Alkaptonuria
- Betalain
- Iodinated tyrosine derivatives
- Pauly reaction
- Tyramine
- Tyrosine sulfation
- Tyrosinemia
References[]
- ^ Jump up to: a b Frey MN, Koetzle TF, Lehmann MS, Hamilton WC (1973). "Precision neutron diffraction structure determination of protein and nucleic acid components. X. A comparison between the crystal and molecular structures of L‐tyrosine and L‐tyrosine hydrochloride". J. Chem. Phys. 58 (6): 2547–2556. Bibcode:1973JChPh..58.2547F. doi:10.1063/1.1679537.
- ^ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. Archived from the original on 9 October 2008. Retrieved 5 March 2018.
- ^ "Tyrosine". The Columbia Electronic Encyclopedia, 6th ed. Infoplease.com — Columbia University Press. 2007. Retrieved 2008-04-20.
- ^ Harper D (2001). "Tyrosine". Online Etymology Dictionary. Retrieved 2008-04-20.
- ^ "Amino Acids - Tyrosine". www.biology.arizona.edu. Retrieved 2018-01-31.
- ^ Pencharz PB, Hsu JW, Ball RO (June 2007). "Aromatic amino acid requirements in healthy human subjects". The Journal of Nutrition. 137 (6 Suppl 1): 1576S–1578S, discussion 1597S-1598S. doi:10.1093/jn/137.6.1576S. PMID 17513429.
- ^ "Tyrosine". University of Maryland Medical Center. Retrieved 2011-03-17.
- ^ Jump up to: a b Top 10 Foods Highest in Tyrosine
- ^ Hoffhines AJ, Damoc E, Bridges KG, Leary JA, Moore KL (December 2006). "Detection and purification of tyrosine-sulfated proteins using a novel anti-sulfotyrosine monoclonal antibody". The Journal of Biological Chemistry. 281 (49): 37877–87. doi:10.1074/jbc.M609398200. PMC 1764208. PMID 17046811.
- ^ Kanan Y, Hamilton RA, Sherry DM, Al-Ubaidi MR (December 2012). "Focus on molecules: sulfotyrosine". Experimental Eye Research. 105: 85–6. doi:10.1016/j.exer.2012.02.014. PMC 3629733. PMID 22406006.
- ^ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ^ Zea-Rey AV, Cruz-Camino H, Vazquez-Cantu DL, Gutiérrez-García VM, Santos-Guzmán J, Cantú-Reyna C (27 November 2017). "The Incidence of Transient Neonatal Tyrosinemia Within a Mexican Population". Journal of Inborn Errors of Metabolism and Screening. 5: 232640981774423. doi:10.1177/2326409817744230.
- ^ Booth AN, Masri MS, Robbins DJ, Emerson OH, Jones FT, DeEds F (1960). "Urinary phenolic acid metabolities of tyrosine". Journal of Biological Chemistry. 235 (9): 2649–2652. doi:10.1016/S0021-9258(19)76930-0.
- ^ Molnár GA, Wagner Z, Markó L, Kó Szegi T, Mohás M, Kocsis B, et al. (November 2005). "Urinary ortho-tyrosine excretion in diabetes mellitus and renal failure: evidence for hydroxyl radical production". Kidney International. 68 (5): 2281–7. doi:10.1111/j.1523-1755.2005.00687.x. PMID 16221230.
- ^ Molnár GA, Nemes V, Biró Z, Ludány A, Wagner Z, Wittmann I (December 2005). "Accumulation of the hydroxyl free radical markers meta-, ortho-tyrosine and DOPA in cataractous lenses is accompanied by a lower protein and phenylalanine content of the water-soluble phase". Free Radical Research. 39 (12): 1359–66. doi:10.1080/10715760500307107. PMID 16298866. S2CID 31154432.
- ^ Humphrey CE, Furegati M, Laumen K, La Vecchia L, Leutert T, Müller-Hartwieg JC, Vögtle M (2007). "Optimized Synthesis of L-m-Tyrosine Suitable for Chemical Scale-Up". Organic Process Research & Development. 11 (6): 1069–1075. doi:10.1021/op700093y.
- ^ Rasmussen DD, Ishizuka B, Quigley ME, Yen SS (October 1983). "Effects of tyrosine and tryptophan ingestion on plasma catecholamine and 3,4-dihydroxyphenylacetic acid concentrations". The Journal of Clinical Endocrinology and Metabolism. 57 (4): 760–3. doi:10.1210/jcem-57-4-760. PMID 6885965.
- ^ Leathwood PD, Pollet P (1982). "Diet-induced mood changes in normal populations". Journal of Psychiatric Research. 17 (2): 147–54. doi:10.1016/0022-3956(82)90016-4. PMID 6764931.
- ^ Jump up to: a b Deijen JB, Orlebeke JF (1994). "Effect of tyrosine on cognitive function and blood pressure under stress". Brain Research Bulletin. 33 (3): 319–23. doi:10.1016/0361-9230(94)90200-3. PMID 8293316. S2CID 33823121.
- ^ Lieberman HR, Corkin S, Spring BJ, Wurtman RJ, Growdon JH (August 1985). "The effects of dietary neurotransmitter precursors on human behavior". The American Journal of Clinical Nutrition. 42 (2): 366–70. doi:10.1093/ajcn/42.2.366. PMID 4025206.
- ^ Jump up to: a b Hao S, Avraham Y, Bonne O, Berry EM (February 2001). "Separation-induced body weight loss, impairment in alternation behavior, and autonomic tone: effects of tyrosine". Pharmacology, Biochemistry, and Behavior. 68 (2): 273–81. doi:10.1016/S0091-3057(00)00448-2. PMID 11267632. S2CID 46405659.
- ^ Magill RA, Waters WF, Bray GA, Volaufova J, Smith SR, Lieberman HR, et al. (August 2003). "Effects of tyrosine, phentermine, caffeine D-amphetamine, and placebo on cognitive and motor performance deficits during sleep deprivation". Nutritional Neuroscience. 6 (4): 237–46. doi:10.1080/1028415031000120552. PMID 12887140. S2CID 21300076.
- ^ Neri DF, Wiegmann D, Stanny RR, Shappell SA, McCardie A, McKay DL (April 1995). "The effects of tyrosine on cognitive performance during extended wakefulness". Aviation, Space, and Environmental Medicine. 66 (4): 313–9. PMID 7794222.
- ^ Reinstein DK, Lehnert H, Wurtman RJ (December 1985). "Dietary tyrosine suppresses the rise in plasma corticosterone following acute stress in rats". Life Sciences. 37 (23): 2157–63. doi:10.1016/0024-3205(85)90566-1. PMID 4068899.
- ^ Deijen JB, Wientjes CJ, Vullinghs HF, Cloin PA, Langefeld JJ (January 1999). "Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course". Brain Research Bulletin. 48 (2): 203–9. doi:10.1016/S0361-9230(98)00163-4. PMID 10230711. S2CID 27927524.
- ^ Mahoney CR, Castellani J, Kramer FM, Young A, Lieberman HR (November 2007). "Tyrosine supplementation mitigates working memory decrements during cold exposure". Physiology & Behavior. 92 (4): 575–82. doi:10.1016/j.physbeh.2007.05.003. PMID 17585971. S2CID 207372821.
- ^ Chinevere TD, Sawyer RD, Creer AR, Conlee RK, Parcell AC (November 2002). "Effects of L-tyrosine and carbohydrate ingestion on endurance exercise performance". Journal of Applied Physiology. 93 (5): 1590–7. doi:10.1152/japplphysiol.00625.2001. PMID 12381742.
- ^ Strüder HK, Hollmann W, Platen P, Donike M, Gotzmann A, Weber K (April 1998). "Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans". Hormone and Metabolic Research. 30 (4): 188–94. doi:10.1055/s-2007-978864. PMID 9623632.
- ^ Thomas JR, Lockwood PA, Singh A, Deuster PA (November 1999). "Tyrosine improves working memory in a multitasking environment". Pharmacology, Biochemistry, and Behavior. 64 (3): 495–500. doi:10.1016/S0091-3057(99)00094-5. PMID 10548261. S2CID 24717770.
- ^ Jump up to: a b Lütke-Eversloh T, Santos CN, Stephanopoulos G (December 2007). "Perspectives of biotechnological production of L-tyrosine and its applications". Applied Microbiology and Biotechnology. 77 (4): 751–62. doi:10.1007/s00253-007-1243-y. PMID 17968539. S2CID 23088822.
- ^ Chavez-Bejar M, Baez-Viveros J, Martinez A, Bolivar F, Gosset G (2012). "Biotechnological production of L-tyrosine and derived compounds". Process Biochemistry. 47 (7): 1017–1026. doi:10.1016/j.procbio.2012.04.005.
External links[]
- Proteinogenic amino acids
- Glucogenic amino acids
- Ketogenic amino acids
- Aromatic amino acids
- Phenols
- Carbonic anhydrase activators