Alkanolamine
Alkanolamines are chemical compounds that contain both hydroxyl (-OH) and amino (-NH2, -NHR, and -NR2) functional groups on an alkane backbone. The term alkanolamine is a broad class term that is sometimes used as a subclassification.[1]
- Alkanolamines
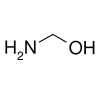
methanolamine, an intermediate in the reaction of ammonia with formaldehyde

[[Ethanolamine] is a commercially useful
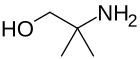
2-amino-2-methyl-1-propanol is a precursor to oxazolines

Sphingosine is a component of somee cell membrane.
1-Aminoalcohols[]
1-Aminoalcohols are better known as hemiaminals. Methanolamine is the simplest member.
2-Aminoalcohols[]
Key members: ethanolamine, dimethylethanolamine, N-methylethanolamine, Aminomethyl propanol
Two popular drugs, often called alkanolamine beta blockers, are members of this structural class: propranolol, pindolol. Isoetarine is yet another medicinally useful derivative of ethanolamine.
2-Aminoalcohols are an important class of organic compounds that are often generated often by the reaction of amines with epoxides. Simple alkanolamines are used as solvents, synthetic intermediates, and high-boiling bases.[2]
Preparation[]
Ethylene oxide and related epoxides add to amines:[2]
- C
2H
4O + R−NH
2 → RNHC
2H
4OH<ref>
Hydrogenation of amino acids gives the corresponding 2-aminoalcohols. Examples include prolinol (from proline) and valinol (from valine).
1,3-, 1,4-, and 1,5-amino alcohols[]
- Heptaminol, a cardiac stimulant
- Propanolamines
Natural products[]
Most proteins and peptides contain both alcohols and amino groups. Two amino acids are alkanolamines, formally speaking: serine and hydroxyproline.
- Veratridine and veratrine
- Tropane alkaloids such as atropine
- hormones and neurotransmitters epinephrine (adrenaline) and norepinephrine (noradrenaline)
References[]
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ Jump up to: a b Matthias Frauenkron; Johann-Peter Melder; Günther Ruider; Roland Rossbacher; Hartmut Höke (2002). "Ethanolamines and Propanolamines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_001. ISBN 3527306730.
External links[]
- Amino+Alcohols at the US National Library of Medicine Medical Subject Headings (MeSH)
- Alcohols
- Amines



