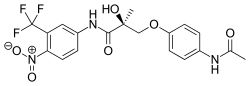
Andarine
 | |
| Clinical data | |
|---|---|
| Other names | Acetamidoxolutamide; Androxolutamide; GTx-007; S-4 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.230.653 |
| Chemical and physical data | |
| Formula | C19H18F3N3O6 |
| Molar mass | 441.363 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Andarine (developmental code names GTx-007, S-4) is an investigational selective androgen receptor modulator (SARM) developed by GTX, Inc for treatment of conditions such as muscle wasting, osteoporosis and benign prostatic hypertrophy,[1] using the nonsteroidal antiandrogen bicalutamide as a lead compound.[2]
Andarine is an orally active partial agonist of the androgen receptor (AR). In intact male rats, 0.5 mg andarine daily was shown to reduce prostate weight to 79.4%, and non-significantly increased levator ani muscle weight. In castrated male rats, this dose restored only 32.5% prostate weight, but 101% levator ani muscle weight [3] This suggests that andarine is able to competitively block binding of dihydrotestosterone to its receptor targets in the prostate gland, but its partial agonist actions at the androgen receptor prevent the side effects associated with the antiandrogens traditionally used for treatment of BPH.[4]
See also[]
- Acetothiolutamide
- Enobosarm
References[]
- ^ Yin, Donghua; Gao, Wenqing; Kearbey, Jeffrey D.; Xu, Huiping; Chung, Kiwon; He, Yali; Marhefka, Craig A.; Veverka, Karen A.; Miller, Duane D.; Dalton, James T. (2002-12-13). "Pharmacodynamics of Selective Androgen Receptor Modulators". Journal of Pharmacology and Experimental Therapeutics. American Society for Pharmacology & Experimental Therapeutics (ASPET). 304 (3): 1334–1340. doi:10.1124/jpet.102.040840. ISSN 0022-3565. PMID 12604714. S2CID 14724811.
- ^ Chen J, Kim J, Dalton JT (June 2005). "Discovery and therapeutic promise of selective androgen receptor modulators". Molecular Interventions. 5 (3): 173–88. doi:10.1124/mi.5.3.7. PMC 2072877. PMID 15994457.
- ^ GGao W, Kearbey JD, Nair VA, Chung K, Parlow AF, Miller DD, Dalton JT (December 2004). "Comparison of the pharmacological effects of a novel selective androgen receptor modulator, the 5alpha-reductase inhibitor finasteride, and the antiandrogen hydroxyflutamide in intact rats: new approach for benign prostate hyperplasia". Endocrinology. 145 (12): 5420–8. doi:10.1210/en.2004-0627. PMC 2098692. PMID 15308613.
- ^ Gao W, Kim J, Dalton JT (August 2006). "Pharmacokinetics and pharmacodynamics of nonsteroidal androgen receptor ligands". Pharmaceutical Research. 23 (8): 1641–58. doi:10.1007/s11095-006-9024-3. PMC 2072875. PMID 16841196.
- Drugs not assigned an ATC code
- Carboxamides
- Phenol ethers
- Selective androgen receptor modulators
- Trifluoromethyl compounds
- Antineoplastic and immunomodulating drug stubs
- Genito-urinary system drug stubs