EM-5854
 | |
| Clinical data | |
|---|---|
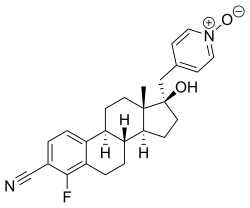
| Other names | 4-Fluoro-17β-hydroxy-17α-[(1-oxidopyridin-1-ium-4-yl)methyl]estra-1,3,5(10)-triene-3-carbonitrile |
| Drug class | Steroidal antiandrogen |
| Identifiers | |
| |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C25H27FN2O2 |
| Molar mass | 406.501 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
EM-5854 is a steroidal antiandrogen which was under development by Endoceutics, Inc. (formerly Endorecherche, Inc.) for the treatment of prostate cancer.[1][2][3][4][5] It was first described in a patent in 2008, and was further characterized in 2012.[2][4] EM-5854 reached phase I/II clinical trials for the treatment of prostate cancer but development was discontinued in March 2019.[1]
The drug acts as a potent and selective competitive antagonist of the androgen receptor (AR).[4][5] Unlike other steroidal antiandrogens like cyproterone acetate, but similarly to nonsteroidal antiandrogens like bicalutamide and enzalutamide, EM-5854 is a pure or silent antagonist of the AR and shows no intrinsic partial androgenic activity.[4] EM-5854 and its metabolite show 3.7-fold and 94-fold higher affinity for the human AR than bicalutamide (0.66% and 17% of the RBA of metribolone, respectively, compared to 0.18% for bicalutamide).[4][5] They also show dramatically increased antiandrogenic potency relative to bicalutamide in in vivo assays.[4][5][6] On the basis of the available research, it has been said that EM-5854 may possibly have 70- to 140-fold the antiandrogenic potency of bicalutamide in humans.[4] EM-5854 and EM-5855 show little to no affinity for other steroid hormone receptors including the estrogen, progesterone, and glucocorticoid receptors.[4] EM-5854 bears a cyano phenyl group, the structural motif of the nonsteroidal antiandrogens.[7]
| Activity | Specifics | Bica | Flu | OH‑Flu | Enza | EM‑5854 | |
|---|---|---|---|---|---|---|---|
| AR RBA (%) | Human | 0.18 | NA | 0.17 | 0.07 | 0.66 | 17 |
| Metri = 100% | Rat | 0.13 | NA | 0.07 | 0.02 | 0.35 | 2.6 |
| Shionogi cells AA activity | Ki (nM) | 81 | NA | NA | 170 | 2.0 | 0.77 |
| LNCaP cells (PSA) AA activity and stim of basal prolif | De50 (nM) (Inhib at 10−7 M (%)) | 1750 (6 ± 10) |
NA | NA | 1380 (−20 ± 3) |
127 (36 ± 7) |
66 (66 ± 1) |
| Stim at 10−7 M (%) | 0 ± 1 | NA | NA | 1 ± 1 | 19 ± 1 | 29 ± 2 | |
| ER RBA (%) | Rat (E2 = 100%) | 0 | NA | 0 | 0 | 0 | 0 |
| PR RBA (%) | Rat (Prom = 100%) | ND | NA | 0 | ND | 0.2 | ND |
| GR RBA (%) | Rat (Dexa = 100%) | 0 | NA | 0 | <0.1 | 0 | 0 |
References[]
- ^ a b "EM 5854 - AdisInsight".
- ^ a b Endorecherche, Inc. Preparation of 17α-substituted steroids as systemic antiandrogens and selective androgen receptor modulators. WO2008124922; 2008 https://patents.google.com/patent/US9284345B2/en
- ^ Zhang X, Lanter JC, Sui Z (September 2009). "Recent advances in the development of selective androgen receptor modulators". Expert Opin Ther Pat. 19 (9): 1239–58. doi:10.1517/13543770902994397. PMID 19505196. S2CID 46186955.
- ^ a b c d e f g h i Gauthier S, Martel C, Labrie F (October 2012). "Steroid derivatives as pure antagonists of the androgen receptor". J. Steroid Biochem. Mol. Biol. 132 (1–2): 93–104. doi:10.1016/j.jsbmb.2012.02.006. PMID 22449547. S2CID 28982450.
- ^ a b c d Cabeza M, Sánchez-Márquez A, Garrido M, Silva A, Bratoeff E (2016). "Recent Advances in Drug Design and Drug Discovery for Androgen- Dependent Diseases". Curr. Med. Chem. 23 (8): 792–815. doi:10.2174/0929867323666160210125642. PMC 5412001. PMID 26861003.
- ^ Salvador JA, Carvalho JF, Neves MA, Silvestre SM, Leitão AJ, Silva MM, Sá e Melo ML (February 2013). "Anticancer steroids: linking natural and semi-synthetic compounds". Nat Prod Rep. 30 (2): 324–74. doi:10.1039/c2np20082a. PMID 23151898.
- ^ Fujii S, Kagechika H (June 2019). "Androgen receptor modulators: a review of recent patents and reports (2012-2018)". Expert Opin Ther Pat. 29 (6): 439–453. doi:10.1080/13543776.2019.1618831. PMID 31092069. S2CID 155103197.
External links[]
- EM-5854 - AdisInsight
- Research programme: androgen receptor antagonists (EM-4350, EM-5855, EM-6537) - AdisInsight
- Abandoned drugs
- Tertiary alcohols
- Estranes
- Experimental cancer drugs
- Fluoroarenes
- Hormonal antineoplastic drugs
- Nitriles
- Prostate cancer
- Pyridinium compounds
- Steroidal antiandrogens
- Genito-urinary system drug stubs
- Antineoplastic and immunomodulating drug stubs