Androstanediol glucuronide
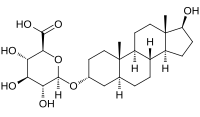
| |
| Names | |
|---|---|
| IUPAC name
17β-Hydroxy-5α-androstan-3α-yl β-D-glucopyranosiduronic acid
| |
| Preferred IUPAC name
(2S,3S,4S,5R,6R)-3,4,5-Trihydroxy-6-{[(1S,3aS,3bR,5aS,7R,9aS,9bS,11aS)-1-hydroxy-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-7-yl]oxy}oxane-2-carboxylic acid | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| Properties | |
| C25H40O8 | |
| Molar mass | 468.587 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
This article may be too technical for most readers to understand. (December 2014) |
3α-Androstanediol glucuronide (3α-ADG) is a metabolite formed from human androgens; compounds involved in the development and maintenance of sexual characteristics. It is formed by the glucuronidation of both dihydrotestosterone and testosterone,[1] and has been proposed as means of measuring androgenic activity.[2]
In women the adrenal steroids, dehydroepiandrosterone sulfate, androstenedione and dehydroepiandrosterone are the major precursors of plasma 3α-ADG, accounting for almost the totality of circulating 3α-ADG. Levels of 3α-ADG decrease significantly with age.[3]
3α-ADG is used as a marker of target tissue cellular action.[citation needed] 3α-ADG correlates with level of 5α-reductase activity (testosterone and 3α-androstanediol to dihydrotestosterone) in the skin. Concentrations of 3α-ADG are associated with the level of cutaneous androgen metabolism.
See also[]
References[]
- ^ Moghissi E, Ablan F, Horton R (September 1984). "Origin of plasma androstanediol glucuronide in men". The Journal of Clinical Endocrinology and Metabolism. 59 (3): 417–21. doi:10.1210/jcem-59-3-417. PMID 6746859.
- ^ Labrie, Fernand; Bélanger, Alain; Bélanger, Patrick; Bérubé, René; Martel, Céline; Cusan, Leonello; Gomez, José; Candas, Bernard; Castiel, Isabelle; Chaussade, Véronique; Deloche, Claire; Leclaire, Jacques (June 2006). "Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women". The Journal of Steroid Biochemistry and Molecular Biology. 99 (4–5): 182–188. doi:10.1016/j.jsbmb.2006.02.004. PMID 16621522. S2CID 31765384.
- ^ Vermeulen A, Giagulli VA (November 1991). "Physiopathology of plasma androstanediol-glucuronide". The Journal of Steroid Biochemistry and Molecular Biology. 39 (5B): 829–33. doi:10.1016/0960-0760(91)90032-z. PMID 1835405. S2CID 46135916.
- 5α-Reduced steroid metabolites
- Androstanes
- Human metabolites
- Glucuronide esters