Ceric ammonium nitrate

| |
| Names | |
|---|---|
| IUPAC name
Diammonium cerium(IV) nitrate
| |
| Other names
Ceric ammonium nitrate (CAN)
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.100 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| H8N8CeO18 | |
| Molar mass | 548.218 g·mol−1 |
| Appearance | orange-red crystals |
| Melting point | 107 to 108 °C (225 to 226 °F; 380 to 381 K) |
| 141 g/100 mL (25 °C) 227 g/100 mL (80 °C) | |
| Structure | |
| Monoclinic | |
| Icosahedral | |
| Hazards | |
| GHS pictograms |   [1] [1]
|
| GHS Signal word | Danger |
GHS hazard statements
|
H272, H302, H315, H319, H335 |
| P220, P261, P305+351+338 | |
| Related compounds | |
Related compounds
|
Ammonium nitrate Cerium(IV) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ceric ammonium nitrate (CAN) is the inorganic compound with the formula (NH4)2Ce(NO3)6. This orange-red, water-soluble cerium salt is a specialised oxidizing agent in organic synthesis and a standard oxidant in quantitative analysis.
Preparation, properties, and structure[]
The anion [Ce(NO
3)
6]2−
is generated by dissolving Ce
2O
3 in hot concentrated HNO3.
The salt consists of the anion [Ce(NO
3)
6]2−
and a pair of NH4+ counter ions. The ammonium ions are not involved in the oxidising reactions of this salt. In the anion each nitrate group chelates the cerium atom in a bidentate manner as shown below:
 Hexanitratocerate anion
Hexanitratocerate anion
The anion [Ce(NO
3)
6]2−
has Th (idealized Oh) molecular symmetry. The CeO12 core defines an icosahedron.[2]
Ce4+ is a strong one-electron oxidizing agent. In terms of its redox potential (E° ~ 1.61 V vs. N.H.E.) it is even stronger oxidizing agent than Cl2 (E° ~ 1.36 V). Few shelf-stable reagents are stronger oxidants. In the redox process Ce(IV) is converted to Ce(III), a one-electron change, signaled by the fading of the solution color from orange to a pale yellow (providing that the substrate and product are not strongly colored).
Applications in organic chemistry[]
In organic synthesis, CAN is useful as an oxidant for many functional groups (alcohols, phenols, and ethers) as well as C–H bonds, especially those that are benzylic. Alkenes undergo dinitroxylation, although the outcome is solvent-dependent. Quinones are produced from catechols and hydroquinones and even nitroalkanes are oxidized.
CAN provides an alternative to the Nef reaction; for example, for synthesis where complicating side reactions usually encountered using other reagents. Oxidative halogenation can be promoted by CAN as an in situ oxidant for benzylic bromination, and the iodination of ketones and uracil derivatives.
For the synthesis of heterocycles[]
Catalytic amounts of aqueous CAN allow the efficient synthesis of quinoxaline derivatives. Quinoxalines are known for their applications as dyes, organic semiconductors, and DNA cleaving agents. These derivatives are also components in antibiotics such as echinomycin and actinomycin. The CAN-catalyzed three-component reaction between anilines and provides an efficient entry into 2-methyl-1,2,3,4-tetrahydroquinolines and the corresponding quinolines obtained by their aromatization.
As a deprotection reagent[]
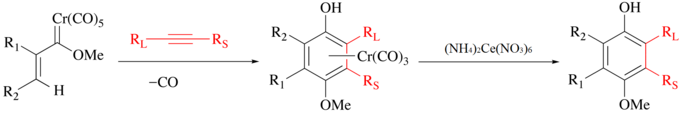
CAN is traditionally used to release organic ligands from metal carbonyls. In the process, the metal is oxidised, CO is evolved, and the organic ligand is released for further manipulation.[3] For example, with the Wulff–Dötz reaction an alkyne, carbon monoxide, and a chromium carbene are combined to form a chromium half-sandwich complex[4][5] and the phenol ligand can be isolated by mild CAN oxidation.
CAN is used to cleave para-methoxybenzyl and 3,4-dimethoxybenzyl ethers, which are protecting groups for alcohols.[6][7] Two equivalents of CAN are required for each equivalent of para-methoxybenzyl ether. The alcohol is released, and the para-methoxybenzyl ether converts to para-methoxybenzaldehyde. The balanced equation is as follows:
- 2 (NH4)2Ce(NO3)6 + H3COC6H4CH2OR + H2O → 4 NH4+ + 2 Ce3+ + 12 NO3− + 2 H+ + H3COC6H4CHO + HOR
Other applications[]
CAN is also a component of chrome etchant,[8] a material that is used in the production of photomasks and liquid crystal displays.[citation needed]
References[]
- ^ Sigma-Aldrich Co., Ammonium cerium(IV) nitrate. Retrieved on 2015-05-13.
- ^ Thomas A. Beineke; J. Delgaudio (1968). "Crystal structure of ceric ammonium nitrate". Inorg. Chem. 7 (4): 715–721. doi:10.1021/ic50062a020.
- ^ L. Brener, J. S. McKennis, and R. Pettit "Cyclobutadiene in Synthesis: endo-Tricyclo[4.4.0.02,5]deca-3,8-diene-7,10-dione" Org. Synth. 1976, 55, 43.doi:10.15227/orgsyn.055.0043
- ^ Waters, M.; Wulff, W. D. (2008). "The Synthesis of Phenols and Quinones via Fischer Carbene Complexes". Organic Reactions. 70 (2): 121–623. doi:10.1002/0471264180.or070.02.
- ^ Dötz, K. H. (1983). "Carbon-Carbon Bond Formation via Carbonyl-Carbene Complexes". Pure and Applied Chemistry. 55 (11). doi:10.1351/pac198355111689.
- ^ Boons, Geert-Jan.; Hale, Karl J. (2000). Organic Synthesis with Carbohydrates (1st ed.) Sheffield, England: Sheffield Academic Press. pp.33
- ^ Kocienski, Phillip J. (1994). Protecting Groups Stuttgart, New York Georg Thieme Verlag. pp 8-9, 52-54
- ^ Walker, Perrin; William H. Tarn (1991). CRC Handbook of Metal Etchants. pp. 287–291. ISBN 0-8493-3623-6.
External links[]
- Ammonium compounds
- Cerium(IV) compounds
- Nitrates
- Coordination compounds
- Oxidizing agents