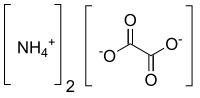
Ammonium oxalate
| |
| Names | |
|---|---|
| IUPAC name
Diammonium ethanedioate
| |
| Other names
Diammonium oxalate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.012.912 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H8N2O4 | |
| Molar mass | 124.096 g·mol−1 |
| Appearance | White solid |
| Melting point | 70 C (158 F, 343.15 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ammonium oxalate, C2H8N2O4 – more commonly written as (NH4)2C2O4 – is an oxalate salt with ammonium (sometimes as a monohydrate). It is a colorless (white) salt under standard conditions and is odorless and non-volatile. It is the ammonium salt of oxalic acid, and occurs in many plants and vegetables.
Vertebrate[]
It is produced in the body of vertebrates by metabolism of glyoxylic acid or ascorbic acid. It is not metabolized but excreted in the urine.[1] It is a constituent of some types of kidney stone.[2][3] It is also found in guano.
Mineralogy[]
Oxammite is a natural, mineral form of ammonium oxalate. This mineral is extremely rare.[4]
Chemistry[]
Ammonium oxalate is used as an analytical reagent and general reducing agent.[1] It and other oxalates are used as anticoagulants, to preserve blood outside the body.
Earth sciences[]
Acid ammonium oxalate (ammonium oxalate acidified to pH 3 with oxalic acid) is commonly employed in soil chemical analysis to extract iron and aluminium from poorly-crystalline minerals (such as ferrihydrite), iron(II)-bearing minerals (such as magnetite) and organic matter.[5][page needed]
References[]
- ^ a b National Center for Biotechnology Information. PubChem Compound Database; CID 14213 (accessed 15 November 2016).
- ^ The International Pharmacopoeia, p.1292, Volume 1, World Health Organization, 2006 ISBN 92-4-156301-X.
- ^ N G Coley, "The collateral sciences in the work of Golding Bird (1814-1854)", Medical History, iss.4, vol.13, October 1969, pp.372.
- ^ http://www.mindat.org
- ^ Rayment, George; Lyons, David (2011). Soil Chemical Methods - Australasia. CSIRO Publishing. ISBN 9780643101364.
- Ammonium compounds
- Anticoagulants
- Oxalates
- Inorganic compound stubs