Dithionate
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dithionate
| |||
| Systematic IUPAC name
Bis(trioxidosulfate)(S—S)(2−)[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| S 2O2− 6 | |||
| Molar mass | 160.126 g mol−1 | ||
| Conjugate acid | Dithionic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
The dithionate (or metabisulfate) anion, S
2O2−
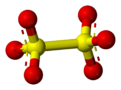
6, is a sulfur oxoanion[2] derived from dithionic acid, H2S2O6. Its chemical formula is sometimes written in a semistructural format, as [O3SSO3]2−. It is the first member of the polythionates.
The sulfur atoms of the dithionate ion are in the +5 oxidation state due to the presence of the S–S bond. Generally, dithionates form stable compounds that are not readily oxidised or reduced. Strong oxidants oxidise them to sulfates and strong reducing agents reduce them to sulfites and dithionites.[3] Aqueous solutions of dithionates are quite stable and can be boiled without decomposition.[4]
The γ-irradiation of crystalline dithionates produces SO•−
3 radical ions.[5] The unpaired electron in the SO•−
3 radical can be detected with electron paramagnetic resonance and barium dithionate has been proposed as the basis for a radiation dosimeter.[6]
The dithionate ion can act as a bidentate ligand.[7]
The structure of the dithionate ion in the solid state is staggered in Na2S2O6·2H2O, whereas in the anhydrous potassium salt it is nearly eclipsed.[3]
Compounds[]
Compounds containing the dithionate ion include:
- sodium dithionate, Na2S2O6
- potassium dithionite, K2S2O6
- , BaS2O6
References[]
- ^ "Dithionate(2−) (CHEBI:29209)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- ^ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ^ Radiation Chemistry of Dithionates G.S. Murthy, R.L. Eager, and K.J. McCallum Can. J. Chem. 49(22),(1971), 3733
- ^ Barium dithionate as an EPR dosemeter Baran M.P., Bugay O.A., Kolesnik S. P., Maksimenko V. M., Teslenko V. V., Petrenko T. L. Desrosiers M. F. Radiation Protection Dosimetry 2006 120, 202; doi:10.1093/rpd/nci531
- ^ Structures of Some Copper (II) Complexes Containing S
2O2−
6 Ion Ishii M. Bulletin of the Yamagata University 5, 1,(2001), 7
External links[]
![]() Media related to Dithionate ion at Wikimedia Commons
Media related to Dithionate ion at Wikimedia Commons
- Dithionates
- Sulfur oxyanions