Fenleuton
 | |
| Clinical data | |
|---|---|
| Trade names | Lofrin |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
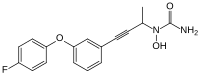
| Formula | C17H15FN2O3 |
| Molar mass | 314.316 g·mol−1 |
| 3D model (JSmol) | |
| |
Fenleuton (trade name Lofrin) is a drug that acts as a 5-lipoxygenase inhibitor and inhibits leukotriene (LTB4, LTC4, LTD4, and LTE4) formation. It has been studied for potential use in veterinary medicine to treat respiratory diseases such as chronic obstructive pulmonary disease (COPD) in horses.[2]
References[]
- ^ "1-{4-[3-(4-Fluorophenoxy)phenyl]-3-butyn-2-yl}-1-hydroxyurea". chemspider.com. Retrieved 24 December 2015.
- ^ Marr KA, Lees P, Page CP, Cunningham FM (1998). "Effect of the 5-lipoxygenase inhibitor, fenleuton, on antigen-induced neutrophil accumulation and lung function changes in horses with chronic obstructive pulmonary disease". J Vet Pharmacol Ther. 21 (3): 241–246. doi:10.1046/j.1365-2885.1998.00127.x. PMID 9673966.
Categories:
- Drugs not assigned an ATC code
- Ureas
- Phenylacetylenes
- Leukotriene pathway inhibitors
- Respiratory system drug stubs