Flavan-3-ol
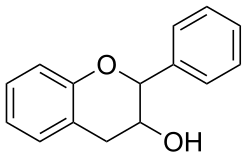


Flavan-3-ols (sometimes referred to as flavanols) are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. These compounds include catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins.
Flavanols (with an "a") are not to be confused with flavonols (with an "o"), a class of flavonoids containing a ketone group.
The single-molecule (monomer) catechin, or isomer epicatechin (see diagram), adds four hydroxyls to flavan-3-ol, making building blocks for concatenated polymers (proanthocyanidins) and higher order polymers (anthocyanidins).[1]
Flavanols possess two chiral carbons, meaning four diastereoisomers occur for each of them.
Catechins are distinguished from the yellow, ketone-containing flavonoids such as quercitin and rutin, which are called flavonols. Early use of the term bioflavonoid was imprecisely applied to include the flavanols, which are distinguished by absence of ketone(s). Catechin monomers, dimers, and trimers (oligomers) are colorless. Higher order polymers, anthocyanidins, exhibit deepening reds and become tannins.[1]
Sources of catechins[]
The catechins are abundant in teas derived from the tea plant Camellia sinensis, as well as in some cocoas and chocolates[2] (made from the seeds of Theobroma cacao). Catechins are also present in the human diet in fruits, vegetables and wine,[3] and are found in many other plant species.[4][5]
Catechin and the gallates[]
Catechin and epicatechin are epimers, with (-)-epicatechin and (+)-catechin being the most common optical isomers found in nature. Catechin was first isolated from the plant extract catechu, from which it derives its name. Heating catechin past its point of decomposition releases pyrocatechol (also called catechol), which explains the common origin of the names of these compounds.
Epigallocatechin and gallocatechin contain an additional phenolic hydroxyl group when compared to epicatechin and catechin, respectively, similar to the difference in pyrogallol compared to pyrocatechol.
Catechin gallates are gallic acid esters of the catechins; an example is epigallocatechin gallate, which is commonly the most abundant catechin in tea.
Metabolism of flavan-3-ols[]
Biosynthesis of (-)-epicatechin[]
The flavonoids are products from a cinnamoyl-CoA starter unit, with chain extension using three molecules of malonyl-CoA. Reactions are catalyzed by a type III PKS enzyme. These enzyme do not use ACPSs, but instead employ coenzyme A esters and have a single active site to perform the necessary series of reactions, e.g. chain extension, condensation, and cyclization. Chain extension of 4-hydroxycinnamoyl-CoA with three molecules of malonyl-CoA gives initially a polyketide (Figure 1), which can be folded. These allow Claisen-like reactions to occur, generating aromatic rings.[6][7]
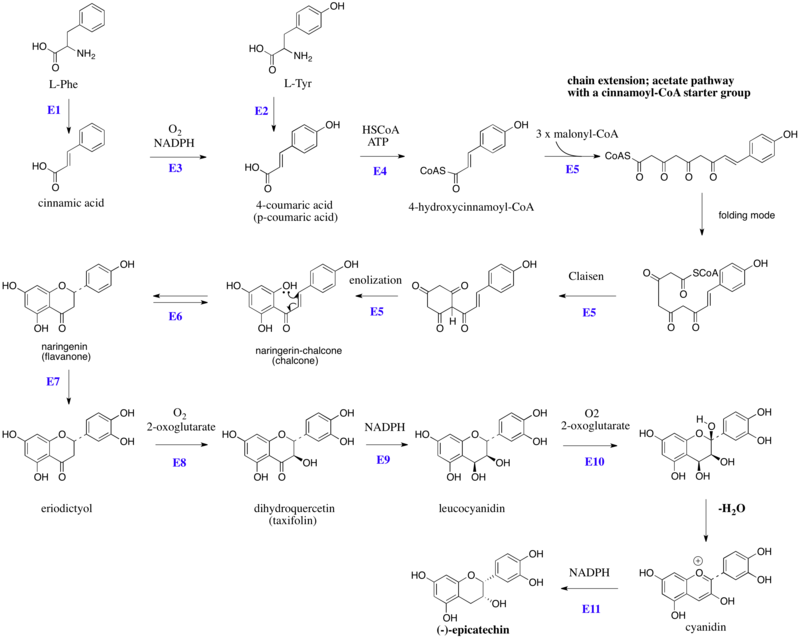
Figure 1:Schematic overview of the flavan-3-ol (-)-epicatechin biosynthesis in plants: Enzymes are indicated in blue, abbreviated as follows: E1, phenylalanine ammonia lyase (PAL), E2, tyrosine ammonia lyase (TAL), E3, cinnamate 4-hydroxylase, E4, 4-coumaroyl: CoA-ligase, E5, chalcone synthase (naringenin-chalcone synthase), E6, chalcone isomerase, E7, Flavonoid 3'-hydroxylase, E8, , E9, dihydroflavanol 4-reductase, E10, anthocyanidin synthase (leucoanthocyanidin dioxygenase), E11, anthocyanidin reductase. HSCoA, Coenzyme A. L-Tyr, L-tyrosine, L-Phe, L-phenylalanine.
Metabolism in humans[]


Most data for human metabolism of flavan-3-ols are available for monomeric compounds, especially Catechin. These compounds are taken up and metabolised upon uptake in the jejunum,[10] mainly by O-methylation and glucuronidation,[11] and then further metabolised by the liver. The colonic microbiome has also an important role in the metabolism of flavan-3-ols and they are catabolised to smaller compounds such as 5-(3′/4′-dihydroxyphenyl)-γ-valerolactones and hippuric acid.[12][8] Only flavan-3-ols with an intact (epi)catechin moiety can be metabolised 5-(3′/4′-dihydroxyphenyl)-γ-valerolactones.[9]
Research and health claims[]
Potential health effects of long-term consumption of flavan-3-ols include reduction of risk factors for cardiovascular diseases, such as decreased levels of blood lipids.[13]
Until 2013, neither the Food and Drug Administration nor the European Food Safety Authority had approved any health claim for catechins or approved any as pharmaceutical drugs.[14][15][16] Moreover, several companies have been cautioned by the FDA over misleading health claims.[17][18]
In 2014, the European Food Safety Authority approved the following health claim for cocoa products containing 200 mg of flavanols and meeting the qualification in dietary supplement products: "cocoa flavanols help maintain the elasticity of blood vessels, which contributes to normal blood flow".[19]
Aglycones[]
| Image | Name | Formula | Oligomers |
|---|---|---|---|
 |
Catechin, C, (+)-Catechin | C15H14O6 | Procyanidins |
 |
Epicatechin, EC, (-)-Epicatechin (cis) | C15H14O6 | Procyanidins |
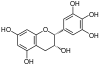 |
Epigallocatechin, EGC | C15H14O7 | Prodelphinidins |
 |
Epicatechin gallate, ECG | C22H18O10 | |
 |
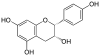
Epigallocatechin gallate, EGCG, (-)-Epigallocatechin gallate |
C22H18O11 | |
 |
Epiafzelechin | C15H14O5 | |
| Fisetinidol | C15H14O5 | ||
| Guibourtinidol | C15H14O4 | Proguibourtinidins | |
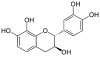 |
Mesquitol | C15H14O6 | |
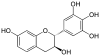 |
Robinetinidol | C15H14O6 | Prorobinetinidins |
Analysis[]
Fluorescence-lifetime imaging microscopy (FLIM) can be used to detect flavanols in plant cells[20]
Other uses[]
Recent study tested catechins employed to coat nanoparticles of iron oxides in the blood. These particles allow visualization of vessels – and especially cancer tumors in mice – in an MRI exam. The nanoparticles would clump together without the catechin coating. [21]
References[]
- ^ Jump up to: a b OPC in Practice, 1995 3rd Edition, by Bert Schwitters in collaboration with Prof. Jack Masquelier.
- ^ Hammerstone JF, Lazarus SA, Schmitz HH (August 2000). "Procyanidin content and variation in some commonly consumed foods". J. Nutr. 130 (8S Suppl): 2086S–92S. doi:10.1093/jn/130.8.2086S. PMID 10917927.
- ^ Ruidavets J, Teissedre P, Ferrières J, Carando S, Bougard G, Cabanis J (November 2000). "Catechin in the Mediterranean diet: vegetable, fruit or wine?". Atherosclerosis. 153 (1): 107–17. doi:10.1016/S0021-9150(00)00377-4. PMID 11058705.
- ^ BBC News | Health | Chocolate 'has health benefits'
- ^ Mabry, Helga; Harborne, J. B.; Mabry, T. J. (1975). The Flavonoids. London: Chapman and Hall. ISBN 978-0-412-11960-6.
- ^ Dewick, Paul M.Medicinal Natural Products: a biosynthetic approach. 3rd ed. John Wiley & Sons Ltd, 2009, p. 168.
- ^ Winkel-Shirley, Brenda.Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. Vol. 126, 2001, p. 485-493.
- ^ Jump up to: a b Ottaviani, Javier I.; Borges, Gina; Momma, Tony Y.; Spencer, Jeremy P. E.; Keen, Carl L.; Crozier, Alan; Schroeter, Hagen (2016-07-01). "The metabolome of [2-14C](−)-epicatechin in humans: implications for the assessment of efficacy, safety and mechanisms of action of polyphenolic bioactives". Scientific Reports. 6 (1): 29034. doi:10.1038/srep29034. ISSN 2045-2322. PMC 4929566. PMID 27363516.
- ^ Jump up to: a b Ottaviani, Javier I.; Fong, Redmond; Kimball, Jennifer; Ensunsa, Jodi L.; Britten, Abigail; Lucarelli, Debora; Luben, Robert; Grace, Philip B.; Mawson, Deborah H. (2018-06-29). "Evaluation at scale of microbiome-derived metabolites as biomarker of flavan-3-ol intake in epidemiological studies". Scientific Reports. 8 (1): 9859. doi:10.1038/s41598-018-28333-w. ISSN 2045-2322. PMC 6026136. PMID 29959422.
- ^ Actis-Goretta, Lucas; Lévèques, Antoine; Rein, Maarit; Teml, Alexander; Schäfer, Christian; Hofmann, Ute; Li, Hequn; Schwab, Matthias; Eichelbaum, Michel (October 2013). "Intestinal absorption, metabolism, and excretion of (-)-epicatechin in healthy humans assessed by using an intestinal perfusion technique". The American Journal of Clinical Nutrition. 98 (4): 924–933. doi:10.3945/ajcn.113.065789. ISSN 1938-3207. PMID 23864538.
- ^ Kuhnle, G.; Spencer, J. P.; Schroeter, H.; Shenoy, B.; Debnam, E. S.; Srai, S. K.; Rice-Evans, C.; Hahn, U. (2000-10-22). "Epicatechin and catechin are O-methylated and glucuronidated in the small intestine". Biochemical and Biophysical Research Communications. 277 (2): 507–512. doi:10.1006/bbrc.2000.3701. ISSN 0006-291X. PMID 11032751.
- ^ Das, N. P. (December 1971). "Studies on flavonoid metabolism. Absorption and metabolism of (+)-catechin in man". Biochemical Pharmacology. 20 (12): 3435–3445. doi:10.1016/0006-2952(71)90449-7. ISSN 0006-2952. PMID 5132890.
- ^ Raman, G; Avendano, EE; Chen, S; Wang, J; Matson, J; Gayer, B; Novotny, JA; Cassidy, A (2019-11-01). "Dietary intakes of flavan-3-ols and cardiometabolic health: systematic review and meta-analysis of randomized trials and prospective cohort studies". The American Journal of Clinical Nutrition. 110 (5): 1067–1078. doi:10.1093/ajcn/nqz178. PMC 6821550. PMID 31504087.
- ^ "FDA approved drug products". US Food and Drug Administration. Retrieved 1 November 2014.
- ^ "Health Claims Meeting Significant Scientific Agreement". US Food and Drug Administration. Retrieved 1 November 2014.
- ^ EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA)2, 3 European Food Safety Authority (EFSA), Parma, Italy (2010). "Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/20061". EFSA Journal. 8 (2): 1489. doi:10.2903/j.efsa.2010.1489.CS1 maint: multiple names: authors list (link)
- ^ "Inspections, Compliance, Enforcement, and Criminal Investigations (Flavonoid Sciences)". US Food and Drug Administration. Retrieved 1 November 2014.
- ^ "Inspections, Compliance, Enforcement, and Criminal Investigations (Unilever, Inc.)". US Food and Drug Administration. Retrieved 31 October 2014.
- ^ "Scientific Opinion on the modification of the authorisation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/20061 following a request in accordance with Article 19 of Regulation (EC) No 1924/2006". EFSA Journal. 12 (5). 2014. doi:10.2903/j.efsa.2014.3654.
- ^ Mueller-Harvey, I.; Feucht, W.; Polster, J.; Trnková, L.; Burgos, P.; Parker, A.W.; Botchway, S.W. (2012). "Two-photon excitation with pico-second fluorescence lifetime imaging to detect nuclear association of flavanols". Anal. Chim. Acta. 719: 68–75. doi:10.1016/j.aca.2011.12.068. PMID 22340533.
- ^ "NEWS-Line for Healthcare Professionals".
External links[]
 Media related to Flavan-3-ols at Wikimedia Commons
Media related to Flavan-3-ols at Wikimedia Commons
- Flavanols
- Nutrients
- Nutrition
- Flavonoid antioxidants
