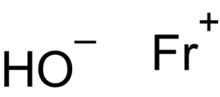
Francium hydroxide
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| Properties | |
| FrHO | |
| Molar mass | 240 g·mol−1 |
| Related compounds | |
Other cations
|
LiOH NaOH KOH RbOH CsOH |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Francium hydroxide is a hypothetical inorganic compound with a chemical formula FrOH. It is francium's hydroxide.
It probably can be produced by reacting francium metal with water:[1]
- 2Fr + 2H2O → 2FrOH + H2↑
This reaction might be explosive.
Francium hydroxide's alkalinity is predicted to be stronger than caesium hydroxide.[2]
References[]
- ^ Robert Krebs (2006), "Francium", The History and Use of Our Earth's Chemical Elements: A Reference Guide, Robert Krebs, p. 64, ISBN 0313334382
- ^ Douglas Considine, Glenn Considine: Van Nostrand’s Scientific Encyclopedia., ISBN 978-1-4757-6920-3, s. 605
Categories:
- Francium compounds
- Hydroxides
- Hypothetical chemical compounds