Orthosilicic acid
| |
| Names | |
|---|---|
| IUPAC name
Orthosilicic acid
| |
| Other names
Silicic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.030.421 |
| EC Number |
|
| 2009 | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H4O4Si | |
| Molar mass | 96.113 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
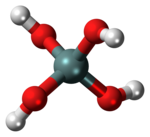
Orthosilicic acid is a chemical compound with formula Si(OH)
4. It has been synthesized using non-aqueous solutions. It is assumed to be present when silicon dioxide (silica) SiO
2 dissolves in water at a millimolar concentration level.
Introduction[]
The term silicic acid has traditionally been used as a synonym for silica, SiO2. Strictly speaking, silica is the anhydride of orthosilicic acid, Si(OH)4.
- Si(OH)4 ⇌ SiO2↓ + 2H2O
The solubility of silicon dioxide in water strongly depends on its crystal structure. The solubility of amorphous silica at the vapor pressure of solutions from 0 to 250 °C and 200 to 1379 bar is given by the equation
- log C = −731/T + 4.52
where C is the silica concentration in mg/kg and T is absolute temperature in kelvins.[1] This equates to a maximum solubility of about 2 mmol/L at ambient temperatures. Attempts to produce more concentrated solutions result in the formation of silica gel.[2] Because the concentration of orthosilicic acid in water is so low, the compounds that are present in solution have not been fully characterized. Linus Pauling predicted that silicic acid would be a very weak acid.[3]
- Si(OH)4 ⇌ Si(OH)3O− + H+
The situation changed in 2017, when the orthosilicic acid monomer was obtained by hydrogenolysis of tetrakis(benzoyloxy)silane, (Si(OCH2C6H5)4, in solution in dimethylacetamide or related solvents. The crystal structure of this compound was determined by X-ray crystallography. Neutron diffraction was also used to determine the location of the hydrogen atoms. Disilicic acid was synthesized by hydrogenation of its hexakis(methylphenoxy) derivative, (CH3C6H4O)3SiOSi(OC6H4CH3)3. Cyclic trisilicic acid, Si3O3(OH)6 and cyclic tetrasilicic acid, Si4O4(OH)8 were synthesized by variations of this method.[4]
With these new discoveries, the term silicic acid has become ambiguous: in addition to the traditional use as a synonym for silica, SiO2, it can now be used for the compound Si(OH)4. The traditional usage is retained in this article for quotes from cited publication which use it.
The derivative Si(OH)3F has been characterized in aqueous solutions containing silicic acid and the fluoride ion.
- Si(OH)4 + F− ⇌ Si(OH)3F + OH−
A fluoride Ion selective electrode was used to determine its stability constant.[5]
Oceanic silicic acid[]
In the uppermost water column the surface ocean is undersaturated with respect to dissolved silica, except for the Antarctic Circumpolar Current south of 55° S. The dissolved silica concentration increases with increasing water depth, and along the conveyor belt from the Atlantic over the Indian into the Pacific Ocean.[6][7]
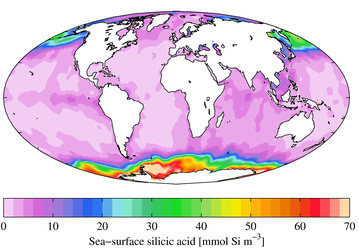
Dissolved silica concentration in the upper pelagic zone.[8]
Theoretical computations indicate that the dissolution of silica in water proceeds begins with the formation of a SiO
2·2H
2O complex, which is converted to orthosilicic acid.[11]
The biogeochemical silica cycle is regulated by the algae known as the diatoms.[12][13] These algae polymerise the silicic acid to so-called biogenic silica, which is used to construct their cell walls (called frustules).[14]
Plants and animals[]
Outside the marine environment compounds of silicon have very little biological function. Small quantities of silica are absorbed from the soil by some plants, to be then excreted in the form of phytoliths.[15]
Subcutaneous injections of orthosilicic acid solutions (around 1%) in mice were found to cause local inflammation and edema. Peritoneal injections of 0.1 mL of freshly prepared acid were often lethal. The toxicity decreased markedly as the solution aged, to the point that after the solution turned to a gel it had no effects other than mechanical ones. The solutions were equally toxic when administered by intravenous injection, but seasoned or gelled solutions were about as toxic as fresh ones.[2]
Research concerning the correlation of aluminium and Alzheimer's disease has included the ability of silicic acid in beer to reduce aluminium uptake in the digestive system as well as to increase renal excretion of aluminium. [16][17]
Choline-stabilized orthosilicic acid (ch-OSA) is a dietary supplement. It has been shown to prevent the loss of tensile strength in human hair;[18] to have a positive effect on the surface and mechanical properties of skin, and on the brittleness of hair and nails;[19] to abate brittle nail syndrome;[20] to partially prevent femoral bone loss in aged ovariectomized rats;[21] to increase the concentration of collagen in calves;[22] and to have a potentially beneficial effect on the formation of collagen in the bones of osteopenic women.[23]
References[]
- ^ Fournier, Robert O.; Rowe, Jack L. (1977). "The solubility of amorphous silica in water at high temperatures and high pressures" (PDF). American Mineralogist. 62: 1052–1056.
- ^ a b Gye, W. E.; Purdy, W. J. (1922). "The Poisonous Properties of Colloidal Silica. I: The Effects of the Parenteral Administration of Large Doses". British Journal of Experimental Pathology. 3 (2): 75–85. PMC 2047780.
- ^ Pauling, Linus (1960). The nature of the chemical bond (3rd. ed.). Ithaka, New York: Cornell University Press. p. 557.
- ^ Igarashi, Masayasu; Matsumoto, Tomohiro; Yagahashi, Fujio; Yamashita, Hiroshi; Ohhara, Takashi; Hanashima, Takayasu; Nakao, Akiko; Moyosh, Taketo; Sato, Kazuhiko; Shimada, Shigeru (2017). "Non-aqueous selective synthesis of orthosilicic acid and its oligomers". Nature Communications. 8 (1): 140. Bibcode:2017NatCo...8..140I. doi:10.1038/s41467-017-00168-5. PMC 5529440. PMID 28747652.
- ^ Ciavatta, Liberato; Iuliano, Mauro; Porto, Raffaella (1988). "Fluorosilicate equilibria in acid solution". Polyhedron. 7 (18): 1773–1779. doi:10.1016/S0277-5387(00)80410-6.
- ^ The figures here have been drawn using the interactive web site which feeds on annual DSi values from LEVITUS94: World Ocean Atlas 1994, an atlas of objectively analyzed fields of major ocean parameters at the annual, seasonal, and monthly time scales. Superseded by WOA98. Edited by Syd Levitus.
- ^ "World Ocean Atlas 1994".
- ^ Information, US Department of Commerce, NOAA National Centers for Environmental. "World Ocean Atlas 2009". www.nodc.noaa.gov. Retrieved 17 April 2018.
- ^ a b The figures here have been drawn using the interactive web site which feeds on annual DSi values from LEVITUS94: World Ocean Atlas 1994, an atlas of objectively analyzed fields of major ocean parameters at the annual, seasonal, and monthly time scales. Superseded by WOA98. Edited by Syd Levitus.
- ^ a b "World Ocean Atlas 1994".
- ^ Mondal, Bhaskar; Ghosh, Deepanwita; Das, Abhijit K. (2009). "Thermochemistry for silicic acid formation reaction: Prediction of new reaction pathway". Chemical Physics Letters. 478 (4–6): 115–119. Bibcode:2009CPL...478..115M. doi:10.1016/j.cplett.2009.07.063.
- ^ Siever, R. (1991). Silica in the oceans: biological-geological interplay. In: Schneider, S. H., Boston, P. H. (eds.), Scientists On Gaia, The MIT Press, Cambridge MA, USA, pp. 287-295.
- ^ Treguer, P., Nelson, D. M., Van Bennekom, A. J., DeMaster, D. J., Leynaert, A. Queguiner, B. (1995). "The silica balance in the world ocean: A reestimate". Science. 268 (5209): 375–379. Bibcode:1995Sci...268..375T. doi:10.1126/science.268.5209.375. PMID 17746543. S2CID 5672525.CS1 maint: uses authors parameter (link)
- ^ Del Amo, Y., and M. A. Brzezinski. 1999. The chemical form of dissolved Si taken up by marine diatoms. J. Phycol. 35:1162-1170. https://onlinelibrary.wiley.com/doi/10.1046/j.1529-8817.1999.3561162.x/abstract
- ^ Snyder, G.H. (2001). "Methods for silicon analysis in plants, soils, and fertilizers". Studies in Plant Science. 8: 185–196. doi:10.1016/S0928-3420(01)80015-X.
- ^ Exley C, Korchazhkina O, Job D, Strekopytov S, Polwart A, Crome P (2006). "Non-invasive therapy to reduce the body burden of aluminium in Alzheimer's disease". J. Alzheimers Dis. 10 (1): 17–24, discussion 29–31. doi:10.3233/jad-2006-10103. PMID 16988476. S2CID 10817452.
- ^ González-Muñoz MJ, Peña A, Meseguer I (2008). "Role of beer as a possible protective factor in preventing Alzheimer's disease". Food Chem. Toxicol. 46 (1): 49–56. doi:10.1016/j.fct.2007.06.036. PMID 17697731.
- ^ Wickett RR, Kossmann E, Barel A, et al. (2007). "Effect of oral intake of choline-stabilized orthosilicic acid on hair tensile strength and morphology in women with fine hair". Arch. Dermatol. Res. 299 (10): 499–505. doi:10.1007/s00403-007-0796-z. PMID 17960402. S2CID 37258319.
- ^ Barel A, Calomme M, Timchenko A, et al. (2005). "Effect of oral intake of choline-stabilized orthosilicic acid on skin, nails and hair in women with photodamaged skin". Arch. Dermatol. Res. 297 (4): 147–53. doi:10.1007/s00403-005-0584-6. PMID 16205932. S2CID 59944678.
- ^ Scheinfeld N, Dahdah MJ, Scher R (2007). "Vitamins and minerals: their role in nail health and disease". J Drugs Dermatol. 6 (8): 782–7. PMID 17763607.
- ^ Calomme M, Geusens P, Demeester N, et al. (2006). "Partial prevention of long-term femoral bone loss in aged ovariectomized rats supplemented with choline-stabilized orthosilicic acid". Calcif. Tissue Int. 78 (4): 227–32. doi:10.1007/s00223-005-0288-0. PMID 16604283. S2CID 19175032.
- ^ Calomme MR, Vanden Berghe DA (1997). "Supplementation of calves with stabilized orthosilicic acid. Effect on the Si, Ca, Mg, and P concentrations in serum and the collagen concentration in skin and cartilage". Biol Trace Elem Res. 56 (2): 153–65. doi:10.1007/BF02785389. PMID 9164661. S2CID 6162455.
- ^ Spector TD, Calomme MR, Anderson SH, et al. (2008). "Choline-stabilized orthosilicic acid supplementation as an adjunct to Calcium/Vitamin D3 stimulates markers of bone formation in osteopenic females: a randomized, placebo-controlled trial". BMC Musculoskelet Disord. 9: 85. doi:10.1186/1471-2474-9-85. PMC 2442067. PMID 18547426.
- Aquatic ecology
- Oceanography
- Oxoacids
- Silicon compounds


