Silica cycle
The silica cycle is the biogeochemical cycle in which biogenic silica is transported between the Earth's systems. Opal silica (SiO2) is a chemical compound of silicon, and is also called silicon dioxide. Silicon is considered a bioessential element and is one of the most abundant elements on Earth.[2][3] The silica cycle has significant overlap with the carbon cycle (see carbonate–silicate cycle) and plays an important role in the sequestration of carbon through continental weathering, biogenic export and burial as oozes on geologic timescales.[4]
Overview[]
| Part of a series on |
| Biogeochemical cycles |
|---|
 |
Silicon, the seventh most abundant element in the universe, is the second most abundant element in the Earth's crust. The weathering of the Earth's crust by CO2-rich rainwater, a key process in the control of atmospheric CO2,[5][6] results in the generation of silicic acid (dSi; Si(OH)4) in aqueous environments. Silicifiers are among the most important aquatic organisms and include micro-organisms (e.g., diatoms, rhizarians, silicoflagellates, several species of choanoflagellates) and macro-organisms (e.g., siliceous sponges). Silicifiers use dSi to precipitate biogenic silica (bSi; SiO2) as internal structures[7] and/or external structures.[8] Phototrophic silicifiers, such as diatoms, globally consume vast amounts of silicon concomitantly with nitrogen (N), phosphorus (P), and inorganic carbon (C), connecting the biogeochemistry of these elements and contributing to the sequestration of atmospheric CO2 in the ocean.[9] Heterotrophic organisms like rhizarians, choanoflagellates, and sponges produce bSi independently of the photoautotrophic processing of C and N.[10][8][11][1]
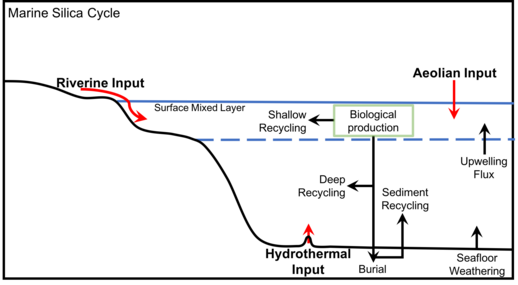
Understanding the silicon cycle is critical for understanding the functioning of marine food webs, biogeochemical cycles, and the biological carbon pump. Silicic acid is delivered to the ocean through six pathways as illustrated in the diagram above, which all ultimately derive from the weathering of the Earth's crust.[12][1]
Terrestrial silica cycling[]

Silica is an important nutrient utilized by plants, trees, and grasses in the terrestrial biosphere. Silicate is transported by rivers and can be deposited in soils in the form of various siliceous polymorphs. Plants can readily uptake silicate in the form of H4SiO4 for the formation of phytoliths. Phytoliths are tiny rigid structures found within plant cells that aid in the structural integrity of the plant.[2] Phytoliths also serve to protect the plants from consumption by herbivores who are unable to consume and digest silica-rich plants efficiently.[2] Silica release from phytolith degradation or dissolution is estimated to occur at a rate double that of global silicate mineral weathering.[3] Considering biogeochemical cycling within ecosystems, the import and export of silica to and from terrestrial ecosystems is small.
Sources[]
Silicate minerals are abundant in rock formations all over the planet, comprising approximately 90% of the Earth's crust.[4] The primary source of silicate to the terrestrial biosphere is weathering. An example of the chemical reaction for this weathering is:
Wollastonite (CaSiO3) and enstatite (MgSiO3) are examples of silicate-based minerals.[22] The weathering process is important for carbon sequestration on geologic timescales.[3][22] The process of and rate of weathering is variable dependent upon rainfall, runoff, vegetation, lithology, and topography.
Sinks[]
The major sink of the terrestrial silica cycle is export to the ocean by rivers. Silica that is stored in plant matter or dissolved can be exported to the ocean by rivers. The rate of this transport is approximately 6 Tmol Si yr−1.[18][3] This is the major sink of the terrestrial silica cycle, as well as the largest source of the marine silica cycle.[18] A minor sink for terrestrial silica is silicate that is deposited in terrestrial sediments and eventually exported to the Earth's crust.
Riverine and aeolian contributions[]
The best estimate for the riverine input (FR) of dSi, based on data representing 60 % of the world river discharge and a discharge-weighted average dSi riverine concentration of 158 µM−Si,[23] remains at FRdSi=6.2 (±1.8) Tmol Si yr−1.[12] However, not only dSi is transferred from the terrestrial to the riverine system, with particulate Si mobilized in crystallized or amorphous forms.[23] According to Saccone and others in 2007,[24] the term “amorphous silica” (aSi) includes biogenic silica (bSi, from phytoliths, freshwater diatoms, sponge spicules), altered bSi, and pedogenic silicates, the three of which can have similar high solubilities and reactivities. Delivery of aSi to the fluvial system has been reviewed by Frings and others in 2016,[25] who suggested a value of FRaSi=1.9(±1.0) Tmol Si yr−1. Therefore, total FR=8.1(±2.0) Tmol Si yr−1.[1]
No progress has been made regarding aeolian dust deposition into the ocean [26] and subsequent release of dSi via dust dissolution in seawater since 2013, when Tréguer and De La Rocha summed the flux of particulate dissolvable silica and wet deposition of dSi through precipitation.[12] Thus, the best estimate for the aeolian flux of dSi, FA, remains 0.5(±0.5) Tmol Si yr−1.[1]
The diagram at the right shows a schematic view of the low-temperature processes that control the dissolution of (either amorphous or crystallized) siliceous minerals in seawater in and to the coastal zone and in the deep ocean, feeding submarine groundwater (FGW) and dissolved silicon in seawater and sediments (FW).[1] These processes correspond to both low and medium energy flux dissipated per volume of a given siliceous particle in the coastal zone, in the continental margins, and in the abysses and to high-energy flux dissipated in the surf zone.[1]
Marine silica cycling[]
Siliceous organisms in the ocean, such as diatoms and radiolaria, are the primary sink of dissolved silicic acid into opal silica.[19] Only 3% of the Si molecules dissolved in the ocean are exported and permanently deposited in marine sediments on the seafloor each year, demonstrating that silicon recycling is a dominant process in the oceans.[3] This rapid recycling is dependent on the dissolution of silica in organic matter in the water column, followed by biological uptake in the photic zone. The estimated residence time of the silica biological reservoir is about 400 years.[3] Opal silica is predominately undersaturated in the world's oceans. This undersaturation promotes rapid dissolution as a result of constant recycling and long residence times. The estimated turnover time of Si is 1.5x104 years.[18] The total net inputs and outputs of silica in the ocean are 9.4 ± 4.7 Tmol Si yr−1 and 9.9 ± 7.3 Tmol Si yr−1, respectively.[18]
Biogenic silica production in the photic zone is estimated to be 240 ± 40 Tmol Si year −1.[18] Dissolution in the surface removes roughly 135 Tmol Si year−1, while the remaining Si is exported to the deep ocean within sinking particles.[3] In the deep ocean, another 26.2 Tmol Si Year−1 is dissolved before being deposited to the sediments as opal rain.[3] Over 90% of the silica here is dissolved, recycled and eventually upwelled for use again in the euphotic zone.[3]
Sources[]
The major sources of marine silica include rivers, groundwater flux, seafloor weathering inputs, hydrothermal vents, and atmospheric deposition (aeolian flux).[22] Rivers are by far the largest source of silica to the marine environment, accounting for up to 90% of all the silica delivered to the ocean.[22][18][27] A source of silica to the marine biological silica cycle is silica that has been recycled by upwelling from the deep ocean and seafloor.
Sinks[]
Deep seafloor deposition is the largest long-term sink of the marine silica cycle (6.3 ± 3.6 Tmol Si year−1), and is roughly balanced by the sources of silica to the ocean.[22] The silica deposited in the deep ocean is primarily in the form of siliceous ooze, which is eventually subducted under the crust and metamorphosed in the upper mantle.[28] Under the mantle, silicate minerals are formed in oozes and eventually uplifted to the surface. At the surface, silica can enter the cycle again through weathering.[28] This process can take tens of millions of years.[28] The only other major sink of silica in the ocean is burial along continental margins (3.6 ± 3.7 Tmol Si year −1), primarily in the form of siliceous sponges.[22] Due to the high degrees of uncertainty in source and sink estimations, it's difficult to conclude if the marine silica cycle is in equilibrium. The residence time of silica in the oceans is estimated to be about 10,000 years.[22] Silica can also be removed from the cycle by becoming chert and being permanently buried.
Anthropogenic influences[]
The rise in agriculture of the past 400 years has increased the exposure rocks and soils, which has resulted in increased rates of silicate weathering. In turn, the leaching of amorphous silica stocks from soils has also increased, delivering higher concentrations of dissolved silica in rivers.[22] Conversely, increased damming has led to a reduction in silica supply to the ocean due to uptake by freshwater diatoms behind dams. The dominance of non-siliceous phytoplankton due to anthropogenic nitrogen and phosphorus loading and enhanced silica dissolution in warmer waters has the potential to limit silicon ocean sediment export in the future.[22]
In 2019 a group of scientists suggested acidification is reducing diatom silica production in the Southern Ocean.[29][30]


Concentration of silicic acid in the upper pelagic zone,[31] showing high levels in the Southern Ocean
Role in climate regulation[]
The silica cycle plays an important role in long term global climate regulation. The global silica cycle also has large effects on the global carbon cycle through the Carbonate-Silicate Cycle.[33] The process of silicate mineral weathering transfers atmospheric CO2 to the hydrologic cycle through the chemical reaction displayed above.[4] Over geologic timescales, the rates of weathering change due to tectonic activity. During a time of high uplift rate, silicate weathering increases which results in high CO2 uptake rates, offsetting increased volcanic CO2 emissions associated with the geologic activity. This balance of weathering and volcanoes is part of what controls the greenhouse effect and ocean pH over geologic time scales.
See also[]
References[]
- ^ a b c d e f g h i Tréguer, Paul J.; Sutton, Jill N.; Brzezinski, Mark; Charette, Matthew A.; Devries, Timothy; Dutkiewicz, Stephanie; Ehlert, Claudia; Hawkings, Jon; Leynaert, Aude; Liu, Su Mei; Llopis Monferrer, Natalia; López-Acosta, María; Maldonado, Manuel; Rahman, Shaily; Ran, Lihua; Rouxel, Olivier (2021). "Reviews and syntheses: The biogeochemical cycle of silicon in the modern ocean". Biogeosciences. 18 (4): 1269–1289. Bibcode:2021BGeo...18.1269T. doi:10.5194/bg-18-1269-2021. S2CID 233993801.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ a b c Hunt, J. W.; Dean, A. P.; Webster, R. E.; Johnson, G. N.; Ennos, A. R. (2008). "A Novel Mechanism by which Silica Defends Grasses Against Herbivory". Annals of Botany. 102 (4): 653–656. doi:10.1093/aob/mcn130. ISSN 1095-8290. PMC 2701777. PMID 18697757.
- ^ a b c d e f g h i j Conley, Daniel J. (December 2002). "Terrestrial ecosystems and the global biogeochemical silica cycle". Global Biogeochemical Cycles. 16 (4): 68–1–68–8. Bibcode:2002GBioC..16.1121C. doi:10.1029/2002gb001894. ISSN 0886-6236.
- ^ a b c Defant, Marc J.; Drummond, Mark S. (October 1990). "Derivation of some modern arc magmas by melting of young subducted lithosphere". Nature. 347 (6294): 662–665. Bibcode:1990Natur.347..662D. doi:10.1038/347662a0. ISSN 0028-0836. S2CID 4267494.
- ^ Garrels, R.M. (1983) "The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years". American Journal of Science, 283: 641-683.
- ^ Wollast, R.; MacKenzie, F. T. (1989). "Global Biogeochemical Cycles and Climate". Climate and Geo-Sciences. pp. 453–473. doi:10.1007/978-94-009-2446-8_26. ISBN 978-0-7923-0412-8.
- ^ Moriceau, Brivaëla; Gehlen, Marion; Tréguer, Paul; Baines, Stephen; Livage, Jacques; André, Luc (2019). "Editorial: Biogeochemistry and Genomics of Silicification and Silicifiers". Frontiers in Marine Science. 6. doi:10.3389/fmars.2019.00057.
- ^ a b Maldonado, Manuel; López-Acosta, María; Sitjà, Cèlia; García-Puig, Marta; Galobart, Cristina; Ercilla, Gemma; Leynaert, Aude (2019). "Sponge skeletons as an important sink of silicon in the global oceans" (PDF). Nature Geoscience. 12 (10): 815–822. Bibcode:2019NatGe..12..815M. doi:10.1038/s41561-019-0430-7. S2CID 201692454.
- ^ Tréguer, Paul; Pondaven, Philippe (2000). "Silica control of carbon dioxide". Nature. 406 (6794): 358–359. doi:10.1038/35019236. PMID 10935620. S2CID 205007880.
- ^ Maldonado, Manuel; Ribes, Marta; Van Duyl, Fleur C. (2012). "Nutrient Fluxes Through Sponges". Advances in Sponge Science: Physiology, Chemical and Microbial Diversity, Biotechnology. Advances in Marine Biology. 62. pp. 113–182. doi:10.1016/B978-0-12-394283-8.00003-5. ISBN 9780123942838. PMID 22664122.
- ^ Llopis Monferrer, Natalia; Boltovskoy, Demetrio; Tréguer, Paul; Sandin, Miguel Méndez; Not, Fabrice; Leynaert, Aude (2020). "Estimating Biogenic Silica Production of Rhizaria in the Global Ocean". Global Biogeochemical Cycles. 34 (3). Bibcode:2020GBioC..3406286L. doi:10.1029/2019GB006286. S2CID 213858837.
- ^ a b c Tréguer, Paul J.; de la Rocha, Christina L. (2013). "The World Ocean Silica Cycle". Annual Review of Marine Science. 5: 477–501. doi:10.1146/annurev-marine-121211-172346. PMID 22809182.
- ^ Sarmiento, Jorge Louis (2006). Ocean biogeochemical dynamics. Gruber, Nicolas. Princeton: Princeton University Press. ISBN 9780691017075. OCLC 60651167.
- ^ Drever, James I. (1993). "The effect of land plants on weathering rates of silicate minerals". Geochimica et Cosmochimica Acta. 58 (10): 2325–2332. doi:10.1016/0016-7037(94)90013-2.
- ^ De La Rocha, Christina; Conley, Daniel J. (2017), "The Venerable Silica Cycle", Silica Stories, Springer International Publishing, pp. 157–176, doi:10.1007/978-3-319-54054-2_9, ISBN 9783319540542
- ^ Chadwick, Oliver A.; Ziegler, Karen; Kurtz, Andrew C.; Derry, Louis A. (2005). "Biological control of terrestrial silica cycling and export fluxes to watersheds". Nature. 433 (7027): 728–731. Bibcode:2005Natur.433..728D. doi:10.1038/nature03299. PMID 15716949. S2CID 4421477.
- ^ Fulweiler, Robinson W.; Carey, Joanna C. (2012-12-31). "The Terrestrial Silica Pump". PLOS ONE. 7 (12): e52932. Bibcode:2012PLoSO...752932C. doi:10.1371/journal.pone.0052932. PMC 3534122. PMID 23300825.
- ^ a b c d e f g h Tréguer, Paul J.; De La Rocha, Christina L. (2013-01-03). "The World Ocean Silica Cycle". Annual Review of Marine Science. 5 (1): 477–501. doi:10.1146/annurev-marine-121211-172346. ISSN 1941-1405. PMID 22809182.
- ^ a b Yool, Andrew; Tyrrell, Toby (2003). "Role of diatoms in regulating the ocean's silicon cycle". Global Biogeochemical Cycles. 17 (4): 14.1–14.22. Bibcode:2003GBioC..17.1103Y. CiteSeerX 10.1.1.394.3912. doi:10.1029/2002GB002018.
- ^ DeMaster, David (2002). "The accumulation and cycling of biogenic silica in the Southern Ocean: revisiting the marine silica budget". Deep Sea Research Part II. 49 (16): 3155–3167. Bibcode:2002DSRII..49.3155D. doi:10.1016/S0967-0645(02)00076-0.
- ^ Sutton, Jill N.; Andre, Luc; Cardinal, Damien; Conley, Daniel J.; de Souza, Gregory F.; Dean, Jonathan; Dodd, Justin; Ehlert, Claudia; Ellwood, Michael J. (2018). "A Review of the Stable Isotope Bio-geochemistry of the Global Silicon Cycle and Its Associated Trace Elements". Frontiers in Earth Science. 5. doi:10.3389/feart.2017.00112. ISSN 2296-6463.
- ^ a b c d e f g h i Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C.J. (July 1999). "Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers". Chemical Geology. 159 (1–4): 3–30. Bibcode:1999ChGeo.159....3G. doi:10.1016/s0009-2541(99)00031-5. ISSN 0009-2541.
- ^ a b Dürr, H. H.; Meybeck, M.; Hartmann, J.; Laruelle, G. G.; Roubeix, V. (2011). "Global spatial distribution of natural riverine silica inputs to the coastal zone". Biogeosciences. 8 (3): 597–620. Bibcode:2011BGeo....8..597D. doi:10.5194/bg-8-597-2011.
- ^ Saccone, L.; Conley, D. J.; Koning, E.; Sauer, D.; Sommer, M.; Kaczorek, D.; Blecker, S. W.; Kelly, E. F. (2007). "Assessing the extraction and quantification of amorphous silica in soils of forest and grassland ecosystems". European Journal of Soil Science. 58 (6): 1446–1459. doi:10.1111/j.1365-2389.2007.00949.x.
- ^ Frings, Patrick J.; Clymans, Wim; Fontorbe, Guillaume; de la Rocha, Christina L.; Conley, Daniel J. (2016). "The continental Si cycle and its impact on the ocean Si isotope budget". Chemical Geology. 425: 12–36. Bibcode:2016ChGeo.425...12F. doi:10.1016/j.chemgeo.2016.01.020.
- ^ Tegen, I. and Kohfeld, K. E. (2006) "Atmospheric Transport of Silicon". In: The Silicon Cycle: Human Perturbations and Impacts on Aquatic Systems, edited by: Ittekot, V., Unger, D., Humborg, C., and Tac An, N. T., 7: 81–91, Island Press.
- ^ Huebner, J. Stephen (November 1982). "Rock-Forming Minerals. Volume 2A: Single-Chain Silicates. W. A. Deer , R. A. Howie , J. Zussman". The Journal of Geology. 90 (6): 748–749. doi:10.1086/628736. ISSN 0022-1376.
- ^ a b c Gaillardet, J.; Dupré, B.; Allègre, C.J. (December 1999). "Geochemistry of large river suspended sediments: silicate weathering or recycling tracer?". Geochimica et Cosmochimica Acta. 63 (23–24): 4037��4051. doi:10.1016/s0016-7037(99)00307-5. ISSN 0016-7037.
- ^ New threat from ocean acidification emerges in the Southern Ocean, Phys.org, 26 August 2019.
- ^ Petrou, K., Baker, K.G., Nielsen, D.A. et al. (2019) "Acidification diminishes diatom silica production in the Southern Ocean". Nature: Climate Change, 9: 781–786. doi:10.1038/s41558-019-0557-y
- ^ Information, US Department of Commerce, NOAA National Centers for Environmental. "World Ocean Atlas 2009". www.nodc.noaa.gov. Retrieved 17 April 2018.
- ^ Tréguer, Paul; Nelson, David M.; Bennekom, Aleido J. Van; DeMaster, David J.; Leynaert, Aude; Quéguiner, Bernard (1995-04-21). "The Silica Balance in the World Ocean: A Reestimate". Science. 268 (5209): 375–379. Bibcode:1995Sci...268..375T. doi:10.1126/science.268.5209.375. ISSN 0036-8075. PMID 17746543. S2CID 5672525.
- ^ Berner, Robert (August 1992). "Weathering, plants, and the long-term carbon cycle". Geochimica et Cosmochimica Acta. 56 (8): 3225–3231. Bibcode:1992GeCoA..56.3225B. doi:10.1016/0016-7037(92)90300-8.
- Biogeochemical cycle
- Silicon