Gallium(III) iodide
| |

| |
| Names | |
|---|---|
| Other names
gallium triiodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.269 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| GaI3 | |
| Molar mass | 450.436 g/mol |
| Appearance | light yellow powder |
| Density | 4.15 g/cm3 |
| Melting point | 212 °C (414 °F; 485 K) |
| Boiling point | 345 °C (653 °F; 618 K) |
| decomposes | |
| −149.0·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
  
| |
Signal word
|
Danger |
| H314, H317, H334, H335, H361 | |
| P280, P305+P351+P338, P310 | |
| NFPA 704 (fire diamond) | 
4
0
1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
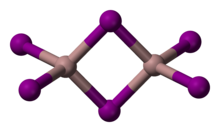
Gallium(III) iodide is the inorganic compound with the formula GaI3. A yellow hygroscopic solid, it is the most common iodide of gallium.[1] In the chemical vapor transport method of growing crystals of gallium arsenide uses iodine as the transport agent. In the solid state, it exists as the dimer Ga2I6.[2]
"Gallium(I) iodide"[]
Gallium triiodide can be reduced with gallium metal to give a green-colored solid called "gallium(I) iodide." The nature of this species is unclear, but it is useful for the preparation of compounds of gallium(I) and gallium(II) and is reported as useful in organic syntheses.[3][4]
See also[]
References[]
- ^ E. Donges (1963). "Gallium(III) Iodide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. 1. NY, NY: Academic Press. p. 846.
- ^ C. Brünig, S. Locmelis, E. Milke, M. Binnewies, "Chemischer Transport fester Lösungen. 27. Mischphasenbildung und chemischer Transport im System ZnSe/GaAs" Zeitschrift für anorganische und allgemeine Chemie 2006, 632, 6 , 1067 - 1072. Brünig, C.; Locmelis, S.; Milke, E.; Binnewies, M. (2006). "Chemischer Transport fester Lösungen. 27. Mischphasenbildung und chemischer Transport im System Zn Se/Ga As". Zeitschrift für Anorganische und Allgemeine Chemie. 632 (6): 1067–1072. doi:10.1002/zaac.200600008.
- ^ Baker, Robert J.; Jones, Cameron. ""GaI": A versatile reagent for the synthetic chemist" Dalton Transactions (2005), (8), pp. 1341-1348. doi:10.1039/b501310k
- ^ GaI: A new reagent for chemo- and diastereoselective C–C bond forming reactions, Green SP, Jones C., Stasch A., Rose R.P, New J. Chem., 2007, 31, 127 - 134, doi:10.1039/b613669a
Categories:
- Iodides
- Gallium compounds
- Metal halides
- Inorganic compound stubs