Gut microbiota
This article's lead section may not adequately summarize its contents. (July 2021) |

Gut microbiota, gut flora, or microbiome are the microorganisms including bacteria, archaea and fungi that live in the digestive tracts of humans[1] and other animals including insects. The gastrointestinal metagenome is the aggregate of all the genomes of gut microbiota.[2][3] The gut is the main location of human microbiota.[4] When the study of gut flora began in 1995, it was thought to have three key roles: a defense against pathogens, maintaining the intestinal epithelium, and metabolizing otherwise indigestible compounds in food.[citation needed] Subsequent studies revealed a role in training the developing immune system and a role in the gut-brain axis.
The microbial composition of the gut microbiota varies across the digestive tract. The colon contains the highest microbial density recorded in any habitat on Earth, representing between 300 and 1000 different species.[5] However, 99% of gut bacteria come from about 30 or 40 species.[6] Bacteria also make up to 60% of the dry mass of feces.[7] Over 99% of the bacteria in the gut are anaerobes, but in the cecum, aerobic bacteria reach high densities.[4] It is estimated that these gut flora have around a hundred times as many genes in total as there are in the human genome.
Overview[]
In humans, the gut microbiota has the largest numbers of bacteria and the greatest number of species compared to other areas of the body.[8] In humans, the gut flora is established at one to two years after birth, by which time the intestinal epithelium and the intestinal mucosal barrier that it secretes have co-developed in a way that is tolerant to, and even supportive of, the gut flora and that also provides a barrier to pathogenic organisms.[9][10]
The relationship between some gut flora and humans is not merely commensal (a non-harmful coexistence), but rather a mutualistic relationship.[4]:700 Some human gut microorganisms benefit the host by fermenting dietary fiber into short-chain fatty acids (SCFAs), such as acetic acid and butyric acid, which are then absorbed by the host.[8][11] Intestinal bacteria also play a role in synthesizing vitamin B and vitamin K as well as metabolizing bile acids, sterols, and xenobiotics.[4][11] The systemic importance of the SCFAs and other compounds they produce are like hormones and the gut flora itself appears to function like an endocrine organ,[11] and dysregulation of the gut flora has been correlated with a host of inflammatory and autoimmune conditions.[8][12]
The composition of human gut microbiota changes over time, when the diet changes, and as overall health changes.[8][12] A systematic review from 2016 examined the preclinical and small human trials that have been conducted with certain commercially available strains of probiotic bacteria and identified those that had the most potential to be useful for certain central nervous system disorders.[13]
Classifications[]
The microbial composition of the gut microbiota varies across the digestive tract. In the stomach and small intestine, relatively few species of bacteria are generally present.[5][14] The colon, in contrast, contains the highest microbial density recorded in any habitat on Earth[15] with up to 1012 cells per gram of intestinal content.[5] These bacteria represent between 300 and 1000 different species.[5][14] However, 99% of the bacteria come from about 30 or 40 species.[6] As a consequence of their abundance in the intestine, bacteria also make up to 60% of the dry mass of feces.[7] Fungi, protists, archaea, and viruses are also present in the gut flora, but less is known about their activities.[16]
Over 99% of the bacteria in the gut are anaerobes, but in the cecum, aerobic bacteria reach high densities.[4] It is estimated that these gut flora have around a hundred times as many genes in total as there are in the human genome.[17]
Many species in the gut have not been studied outside of their hosts because most cannot be cultured.[14][6][18] While there are a small number of core species of microbes shared by most individuals, populations of microbes can vary widely among different individuals.[19] Within an individual, microbe populations stay fairly constant over time, even though some alterations may occur with changes in lifestyle, diet and age.[5][20] The Human Microbiome Project has set out to better describe the microflora of the human gut and other body locations.
The four dominant bacterial phyla in the human gut are Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria.[21] Most bacteria belong to the genera Bacteroides, Clostridium, Faecalibacterium,[5][6] Eubacterium, Ruminococcus, Peptococcus, Peptostreptococcus, and Bifidobacterium.[5][6] Other genera, such as Escherichia and Lactobacillus, are present to a lesser extent.[5] Species from the genus Bacteroides alone constitute about 30% of all bacteria in the gut, suggesting that this genus is especially important in the functioning of the host.[14]
Fungal genera that have been detected in the gut include Candida, Saccharomyces, Aspergillus, Penicillium, Rhodotorula, Trametes, Pleospora, Sclerotinia, Bullera, and Galactomyces, among others.[22][23] Rhodotorula is most frequently found in individuals with inflammatory bowel disease while Candida is most frequently found in individuals with hepatitis B cirrhosis and chronic hepatitis B.[22]
Archaea constitute another large class of gut flora which are important in the metabolism of the bacterial products of fermentation.
Industralization is associated with changes in the microbiota and the reduction of diversity could drive certain species to extinction; in 2018, researchers proposed a biobank repository of human microbiota.[24]
Enterotype[]
An enterotype is a classification of living organisms based on its bacteriological ecosystem in the human gut microbiome not dictated by age, gender, body weight, or national divisions.[25] There are indications that long-term diet influences enterotype.[26] Three human enterotypes have been proposed,[25][27] but their value has been questioned.[28]
Composition[]
Bacteriome[]
Stomach[]
Due to the high acidity of the stomach, most microorganisms cannot survive there. The main bacterial inhabitants of the stomach include: Streptococcus, Staphylococcus, Lactobacillus, Peptostreptococcus.[4]:720 Helicobacter pylori is a gram-negative spiral bacterium that establishes on gastric mucosa causing chronic gastritis, and peptic ulcer disease, and is a carcinogen for gastric cancer.[4]:904
Intestines[]
| Bacteria commonly found in the human colon[29] | |
| Bacterium | Incidence (%) |
|---|---|
| Bacteroides fragilis | 100 |
| Bacteroides melaninogenicus | 100 |
| 100 | |
| Enterococcus faecalis | 100 |
| Escherichia coli | 100 |
| Enterobacter sp. | 40–80 |
| Klebsiella sp. | 40–80 |
| Bifidobacterium bifidum | 30–70 |
| Staphylococcus aureus | 30–50 |
| Lactobacillus | 20–60 |
| Clostridium perfringens | 25–35 |
| Proteus mirabilis | 5–55 |
| Clostridium tetani | 1–35 |
| Clostridium septicum | 5–25 |
| Pseudomonas aeruginosa | 3–11 |
| Salmonella enterica | 3–7 |
| Faecalibacterium prausnitzii | ?common |
| Peptostreptococcus sp. | ?common |
| Peptococcus sp. | ?common |
The small intestine contains a trace amount of microorganisms due to the proximity and influence of the stomach. Gram-positive cocci and rod-shaped bacteria are the predominant microorganisms found in the small intestine.[4] However, in the distal portion of the small intestine alkaline conditions support gram-negative bacteria of the Enterobacteriaceae.[4] The bacterial flora of the small intestine aid in a wide range of intestinal functions. The bacterial flora provide regulatory signals that enable the development and utility of the gut. Overgrowth of bacteria in the small intestine can lead to intestinal failure.[30] In addition the large intestine contains the largest bacterial ecosystem in the human body.[4] About 99% of the large intestine and feces flora are made up of obligate anaerobes such as Bacteroides and Bifidobacterium.[31] Factors that disrupt the microorganism population of the large intestine include antibiotics, stress, and parasites.[4]
Bacteria make up most of the flora in the colon[32] and 60% of the dry mass of feces.[5] This fact makes feces an ideal source of gut flora for any tests and experiments by extracting the nucleic acid from fecal specimens, and bacterial 16S rRNA gene sequences are generated with bacterial primers. This form of testing is also often preferable to more invasive techniques, such as biopsies.
Five phyla dominate the intestinal microbiota: bacteroidetes, firmicutes, actinobacteria, proteobacteria, and verrucomicrobia—with bacteroidetes and firmicutes constituting 90% of the composition.[33] Somewhere between 300[5] and 1000 different species live in the gut,[14] with most estimates at about 500.[34][35] However, it is probable that 99% of the bacteria come from about 30 or 40 species, with Faecalibacterium prausnitzii (phylum firmicutes) being the most common species in healthy adults.[6][36]
Research suggests that the relationship between gut flora and humans is not merely commensal (a non-harmful coexistence), but rather is a mutualistic, symbiotic relationship.[14] Though people can survive with no gut flora,[34] the microorganisms perform a host of useful functions, such as fermenting unused energy substrates, training the immune system via end products of metabolism like propionate and acetate, preventing growth of harmful species, regulating the development of the gut, producing vitamins for the host (such as biotin and vitamin K), and producing hormones to direct the host to store fats.[4] Extensive modification and imbalances of the gut microbiota and its microbiome or gene collection are associated with obesity.[37] However, in certain conditions, some species are thought to be capable of causing disease by causing infection or increasing cancer risk for the host.[5][32]
Mycobiome[]
Fungi and protists also make up a part of the gut flora, but less is known about their activities.[38]
Virome[]
The human virome is mostly bacteriophages.[39]
Variation[]
Age[]
It has been demonstrated that there are common patterns of microbiome composition evolution during life.[40] In general, the diversity of microbiota composition of fecal samples is significantly higher in adults than in children, although interpersonal differences are higher in children than in adults.[41] Much of the maturation of microbiota into an adult-like configuration happens during the three first years of life.[41]
As the microbiome composition changes, so does the composition of bacterial proteins produced in the gut. In adult microbiomes, a high prevalence of enzymes involved in fermentation, methanogenesis and the metabolism of arginine, glutamate, aspartate and lysine have been found. In contrast, in infant microbiomes the dominant enzymes are involved in cysteine metabolism and fermentation pathways.[41]
Diet[]
Studies and statistical analyses have identified the different bacterial genera in gut microbiota and their associations with nutrient intake. Gut microflora is mainly composed of three enterotypes: Prevotella, Bacteroides, and Ruminococcus. There is an association between the concentration of each microbial community and diet. For example, Prevotella is related to carbohydrates and simple sugars, while Bacteroides is associated with proteins, amino acids, and saturated fats. Specialist microbes that break down mucin survive on their host's carbohydrate excretions.[42] One enterotype will dominate depending on the diet. Altering the diet will result in a corresponding change in the numbers of species.[26] A 2021 study suggests that childhood diet and exercise can substantially affect adult microbiome composition and diversity. The authors show that mice on a high-fat diet as juveniles have lower bacterial diversity as adults after a washout period equivalent to six human years.[43][44][45]
Vegetarian and vegan diets[]
While plant-based diets have some variation, vegetarian and vegan diets patterns are the most common. Vegetarian diets exclude meat products (which include fish) but still allow for eggs and dairy, while vegan diets exclude all forms of animal products. The diets of vegetarian and vegan individuals create a microbiome distinct from meat eaters, however there is not a significant distinction between the two.[46][unreliable medical source?] In diets that are centered around meat and animal products, there are high abundances of Alistipes, Bilophila and Bacteroides which are all bile tolerant and may promote inflammation in the gut. In this type of diet, the group Firmicutes, which is associated with the metabolism of dietary plant polysaccharides, is found in low concentrations.[47] Conversely, diets rich in plant-based materials are associated with greater diversity in the gut microbiome overall, and have a greater abundance of Prevotella, responsible for the long-term processing of fibers, rather than the bile tolerant species.[48][unreliable medical source?] Diet can be used to alter the composition of the gut microbiome in relatively short timescales. However, if wanting to change the microbiome to combat a disease or illness, long-term changes in diet have proven to be most successful.[47]
Geography[]
Gut microbiome composition depends on the geographic origin of populations. Variations in a trade-off of Prevotella, the representation of the urease gene, and the representation of genes encoding glutamate synthase/degradation or other enzymes involved in amino acids degradation or vitamin biosynthesis show significant differences between populations from the US, Malawi or Amerindian origin.[41]
The US population has a high representation of enzymes encoding the degradation of glutamine and enzymes involved in vitamin and lipoic acid biosynthesis; whereas Malawi and Amerindian populations have a high representation of enzymes encoding glutamate synthase and they also have an overrepresentation of α-amylase in their microbiomes. As the US population has a diet richer in fats than Amerindian or Malawian populations which have a corn-rich diet, the diet is probably the main determinant of the gut bacterial composition.[41]
Further studies have indicated a large difference in the composition of microbiota between European and rural African children. The fecal bacteria of children from Florence were compared to that of children from the small rural village of Boulpon in Burkina Faso. The diet of a typical child living in this village is largely lacking in fats and animal proteins and rich in polysaccharides and plant proteins. The fecal bacteria of European children were dominated by Firmicutes and showed a marked reduction in biodiversity, while the fecal bacteria of the Boulpon children was dominated by Bacteroidetes. The increased biodiversity and different composition of gut flora in African populations may aid in the digestion of normally indigestible plant polysaccharides and also may result in a reduced incidence of non-infectious colonic diseases.[49]
On a smaller scale, it has been shown that sharing numerous common environmental exposures in a family is a strong determinant of individual microbiome composition. This effect has no genetic influence and it is consistently observed in culturally different populations.[41]
Malnourishment[]
Malnourished children have less mature and less diverse gut microbiota than healthy children, and changes in the microbiome associated with nutrient scarcity can in turn be a pathophysiological cause of malnutrition.[50][51] Malnourished children also typically have more potentially pathogenic gut flora, and more yeast in their mouths and throats.[52] Altering diet may lead to changes in gut microbiota composition and diversity.[42]
Race and ethnicity[]
Researchers with the American Gut Project and Human Microbiome Project found that twelve microbe families varied in abundance based on the race or ethnicity of the individual. The strength of these associations is limited by the small sample size: the American Gut Project collected data from 1,375 individuals, 90% of whom were white.[53] The Healthy Life in an Urban Setting (HELIUS) study in Amsterdam found that those of Dutch ancestry had the highest level of gut microbiota diversity, while those of South Asian and Surinamese descent had the lowest diversity. The study results suggested that individuals of the same race or ethnicity have more similar microbiomes than individuals of different racial backgrounds.[53]
Socioeconomic status[]
As of 2020, at least two studies have demonstrated a link between an individual's socioeconomic status (SES) and their gut microbiota. A study in Chicago found that individuals in higher SES neighborhoods had greater microbiota diversity. People from higher SES neighborhoods also had more abundant Bacteroides bacteria. Similarly, a study of twins in the United Kingdom found that higher SES was also linked with a greater gut diversity.[53]
Acquisition in human infants[]
The establishment of a gut flora is crucial to the health of an adult, as well as the functioning of the gastrointestinal tract.[54] In humans, a gut flora similar to an adult's is formed within one to two years of birth as microbiota are acquired through parent-to-child transmission and transfer from food, water, and other environmental sources.[55][9]
The traditional view of the gastrointestinal tract of a normal fetus is that it is sterile, although this view has been challenged in the past few years.[56] Multiple lines of evidence have begun to emerge that suggest there may be bacteria in the intrauterine environment. In humans, research has shown that microbial colonization may occur in the fetus[57] with one study showing Lactobacillus and Bifidobacterium species were present in placental biopsies.[58] Several rodent studies have demonstrated the presence of bacteria in the amniotic fluid and placenta, as well as in the meconium of babies born by sterile cesarean section.[59][60] In another study, researchers administered a culture of bacteria orally to a pregnant dam, and detected the bacteria in the offspring, likely resulting from transmission between the digestive tract and amniotic fluid via the blood stream.[61] However, researchers caution that the source of these intrauterine bacteria, whether they are alive, and their role, is not yet understood.[62][58]
During birth and rapidly thereafter, bacteria from the mother and the surrounding environment colonize the infant's gut.[9] The exact sources of bacteria is not fully understood, but may include the birth canal, other people (parents, siblings, hospital workers), breastmilk, food, and the general environment with which the infant interacts.[63] However, as of 2013, it remains unclear whether most colonizing arises from the mother or not.[9] Infants born by caesarean section may also be exposed to their mothers' microflora, but the initial exposure is most likely to be from the surrounding environment such as the air, other infants, and the nursing staff, which serve as vectors for transfer.[57] During the first year of life, the composition of the gut flora is generally simple and changes a great deal with time and is not the same across individuals.[9] The initial bacterial population are generally facultative anaerobic organisms; investigators believe that these initial colonizers decrease the oxygen concentration in the gut, which in turn allows obligately anaerobic bacteria like Bacteroides, Actinobacteria, and Firmicutes to become established and thrive.[9] Breast-fed babies become dominated by bifidobacteria, possibly due to the contents of bifidobacterial growth factors in breast milk, and by the fact that breast milk carries prebiotic components, allowing for healthy bacterial growth.[58][64] In contrast, the microbiota of formula-fed infants is more diverse, with high numbers of Enterobacteriaceae, enterococci, bifidobacteria, Bacteroides, and clostridia.[65]
Caesarean section, antibiotics, and formula feeding may alter the gut microbiome composition.[58] Children treated with antibiotics have less stable, and less diverse floral communities.[66] Caesarean sections have been shown to be disruptive to mother-offspring transmission of bacteria, which impacts the overall health of the offspring by raising risks of disease such as celiacs, asthma, and type 1 diabetes.[58] This further evidences the importance of a healthy gut microbiome. Various methods of microbiome restoration are being explored, typically involving exposing the infant to maternal vaginal contents, and oral probiotics.[58]
Functions[]
When the study of gut flora began in 1995,[67] it was thought to have three key roles: direct defense against pathogens, fortification of host defense by its role in developing and maintaining the intestinal epithelium and inducing antibody production there, and metabolizing otherwise indigestible compounds in food; subsequent work discovered its role in training the developing immune system, and yet further work focused on its role in the gut-brain axis.[68]
Direct inhibition of pathogens[]
The gut flora community plays a direct role in defending against pathogens by fully colonising the space, making use of all available nutrients, and by secreting compounds that kill or inhibit unwelcome organisms that would compete for nutrients with it, these compounds are known as cytokines.[69] Different strains of gut bacteria cause the production of different cytokines. Cytokines are chemical compounds produced by our immune system for initiating the inflammatory response against infections. Disruption of the gut flora allows competing organisms like Clostridium difficile to become established that otherwise are kept in abeyance.[69]
Development of enteric protection and immune system[]
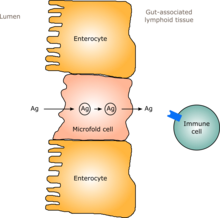
In humans, a gut flora similar to an adult's is formed within one to two years of birth.[9] As the gut flora gets established, the lining of the intestines – the intestinal epithelium and the intestinal mucosal barrier that it secretes – develop as well, in a way that is tolerant to, and even supportive of, commensalistic microorganisms to a certain extent and also provides a barrier to pathogenic ones.[9] Specifically, goblet cells that produce the mucosa proliferate, and the mucosa layer thickens, providing an outside mucosal layer in which "friendly" microorganisms can anchor and feed, and an inner layer that even these organisms cannot penetrate.[9][10] Additionally, the development of gut-associated lymphoid tissue (GALT), which forms part of the intestinal epithelium and which detects and reacts to pathogens, appears and develops during the time that the gut flora develops and established.[9] The GALT that develops is tolerant to gut flora species, but not to other microorganisms.[9] GALT also normally becomes tolerant to food to which the infant is exposed, as well as digestive products of food, and gut flora's metabolites (molecules formed from metabolism) produced from food.[9]
The human immune system creates cytokines that can drive the immune system to produce inflammation in order to protect itself, and that can tamp down the immune response to maintain homeostasis and allow healing after insult or injury.[9] Different bacterial species that appear in gut flora have been shown to be able to drive the immune system to create cytokines selectively; for example Bacteroides fragilis and some Clostridia species appear to drive an anti-inflammatory response, while some segmented filamentous bacteria drive the production of inflammatory cytokines.[9][70] Gut flora can also regulate the production of antibodies by the immune system.[9][71] One function of this regulation is to cause B cells to class switch to IgA. In most cases B cells need activation from T helper cells to induce class switching; however, in another pathway, gut flora cause NF-kB signaling by intestinal epithelial cells which results in further signaling molecules being secreted.[72] These signaling molecules interact with B cells to induce class switching to IgA.[72] IgA is an important type of antibody that is used in mucosal environments like the gut. It has been shown that IgA can help diversify the gut community and helps in getting rid of bacteria that cause inflammatory responses.[73] Ultimately, IgA maintains a healthy environment between the host and gut bacteria.[73] These cytokines and antibodies can have effects outside the gut, in the lungs and other tissues.[9]
The immune system can also be altered due to the gut bacteria's ability to produce metabolites that can affect cells in the immune system. For example short-chain fatty acids (SCFA) can be produced by some gut bacteria through fermentation.[74] SCFAs stimulate a rapid increase in the production of innate immune cells like neutrophils, basophils and eosinophils.[74] These cells are part of the innate immune system that try to limit the spread of infection.
Metabolism[]
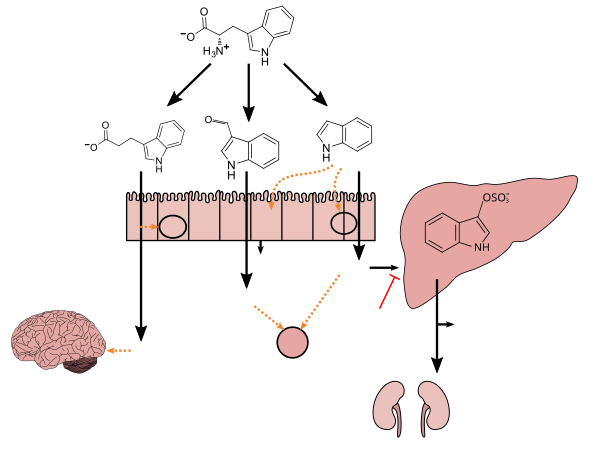
Tryptophan metabolism by human gastrointestinal microbiota ()
|
Without gut flora, the human body would be unable to utilize some of the undigested carbohydrates it consumes, because some types of gut flora have enzymes that human cells lack for breaking down certain polysaccharides.[11] Rodents raised in a sterile environment and lacking in gut flora need to eat 30% more calories just to remain the same weight as their normal counterparts.[11] Carbohydrates that humans cannot digest without bacterial help include certain starches, fiber, oligosaccharides, and sugars that the body failed to digest and absorb like lactose in the case of lactose intolerance and sugar alcohols, mucus produced by the gut, and proteins.[8][11]
Bacteria turn carbohydrates they ferment into short-chain fatty acids by a form of fermentation called .[35] Products include acetic acid, propionic acid and butyric acid.[6][35] These materials can be used by host cells, providing a major source of energy and nutrients.[35] Gases (which are involved in signaling[79] and may cause flatulence) and organic acids, such as lactic acid, are also produced by fermentation.[6] Acetic acid is used by muscle, propionic acid facilitates liver production of ATP, and butyric acid provides energy to gut cells.[35]
Gut flora also synthesize vitamins like biotin and folate, and facilitate absorption of dietary minerals, including magnesium, calcium, and iron.[5][20] Methanobrevibacter smithii is unique because it is not a species of bacteria, but rather a member of domain Archeae, and is the most abundant methane-producing archaeal species in the human gastrointestinal microbiota.[80]
Gut microbiota also serve as a source of Vitamins K and B12 that are not produced by the body or produced in little amount.[81][82]
Pharmacomicrobiomics[]
The human metagenome (i.e., the genetic composition of an individual and all microorganisms that reside on or within the individual's body) varies considerably between individuals.[83][84] Since the total number of microbial and viral cells in the human body (over 100 trillion) greatly outnumbers Homo sapiens cells (tens of trillions),[note 1][83][85] there is considerable potential for interactions between drugs and an individual's microbiome, including: drugs altering the composition of the human microbiome, drug metabolism by microbial enzymes modifying the drug's pharmacokinetic profile, and microbial drug metabolism affecting a drug's clinical efficacy and toxicity profile.[83][84][86]
Apart from carbohydrates, gut microbiota can also metabolize other xenobiotics such as drugs, phytochemicals, and food toxicants. More than 30 drugs have been shown to be metabolized by gut microbiota.[87] The microbial metabolism of drugs can sometimes inactivate the drug.[88]
Gut-brain axis[]
The gut-brain axis is the biochemical signaling that takes place between the gastrointestinal tract and the central nervous system.[68] That term has been expanded to include the role of the gut flora in the interplay; the term "microbiome-gut-brain axis" is sometimes used to describe paradigms explicitly including the gut flora.[68][89][90] Broadly defined, the gut-brain axis includes the central nervous system, neuroendocrine and neuroimmune systems including the hypothalamic–pituitary–adrenal axis (HPA axis), sympathetic and parasympathetic arms of the autonomic nervous system including the enteric nervous system, the vagus nerve, and the gut microbiota.[68][90]
A systematic review from 2016 examined the preclinical and small human trials that have been conducted with certain commercially available strains of probiotic bacteria and found that among those tested, Bifidobacterium and Lactobacillus genera (B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei), had the most potential to be useful for certain central nervous system disorders.[13]
Alterations in microbiota balance[]
Effects of antibiotic use[]
Altering the numbers of gut bacteria, for example by taking broad-spectrum antibiotics, may affect the host's health and ability to digest food.[91] Antibiotics can cause antibiotic-associated diarrhea by irritating the bowel directly, changing the levels of microbiota, or allowing pathogenic bacteria to grow.[6] Another harmful effect of antibiotics is the increase in numbers of antibiotic-resistant bacteria found after their use, which, when they invade the host, cause illnesses that are difficult to treat with antibiotics.[91]
Changing the numbers and species of gut microbiota can reduce the body's ability to ferment carbohydrates and metabolize bile acids and may cause diarrhea. Carbohydrates that are not broken down may absorb too much water and cause runny stools, or lack of SCFAs produced by gut microbiota could cause diarrhea.[6]
A reduction in levels of native bacterial species also disrupts their ability to inhibit the growth of harmful species such as C. difficile and , and these species can get out of hand, though their overgrowth may be incidental and not be the true cause of diarrhea.[5][6][91] Emerging treatment protocols for C. difficile infections involve fecal microbiota transplantation of donor feces (see Fecal transplant).[92] Initial reports of treatment describe success rates of 90%, with few side effects. Efficacy is speculated to result from restoring bacterial balances of bacteroides and firmicutes classes of bacteria.[93]
The composition of the gut microbiome also changes in severe illnesses, due not only to antibiotic use but also to such factors as ischemia of the gut, failure to eat, and immune compromise. Negative effects from this have led to interest in , a treatment to kill only pathogenic bacteria and allow the re-establishment of healthy ones.[94]
Antibiotics alter the population of the microbiota in the gastrointestinal tract, and this may change the intra-community metabolic interactions, modify caloric intake by using carbohydrates, and globally affects host metabolic, hormonal and immune homeostasis.[95]
There is reasonable evidence that taking probiotics containing Lactobacillus species may help prevent antibiotic-associated diarrhea and that taking probiotics with Saccharomyces (e.g., Saccharomyces boulardii ) may help to prevent Clostridium difficile infection following systemic antibiotic treatment.[96]
Pregnancy[]
The gut microbiota of a woman changes as pregnancy advances, with the changes similar to those seen in metabolic syndromes such as diabetes. The change in gut microbiota causes no ill effects. The newborn's gut microbiota resemble the mother's first-trimester samples. The diversity of the microbiome decreases from the first to third trimester, as the numbers of certain species go up.[58][97]
Probiotics, prebiotics, synbiotics, and pharmabiotics[]
Probiotics are microorganisms that are believed to provide health benefits when consumed.[98][99] With regard to gut microbiota, prebiotics are typically non-digestible, fiber compounds that pass undigested through the upper part of the gastrointestinal tract and stimulate the growth or activity of advantageous gut flora by acting as substrate for them.[35][100]
Synbiotics refers to food ingredients or dietary supplements combining probiotics and prebiotics in a form of synergism.[101]
The term "pharmabiotics" is used in various ways, to mean: pharmaceutical formulations (standardized manufacturing that can obtain regulatory approval as a drug) of probiotics, prebiotics, or synbiotics;[102] probiotics that have been genetically engineered or otherwise optimized for best performance (shelf life, survival in the digestive tract, etc.);[103] and the natural products of gut flora metabolism (vitamins, etc.).[104]
There is some evidence that treatment with some probiotic strains of bacteria may be effective in irritable bowel syndrome and chronic idiopathic constipation. Those organisms most likely to result in a decrease of symptoms have included:
- Enterococcus faecium
- Lactobacillus plantarum
- Lactobacillus rhamnosus
- Propionibacterium freudenreichii
- Bifidobacterium breve
- Lactobacillus reuteri
- Lactobacillus salivarius
- Bifidobacterium infantis
- Streptococcus thermophilus[105][106][107]
Research[]
Tests for whether non-antibiotic drugs may impact human gut-associated bacteria were performed by in vitro analysis on more than 1000 marketed drugs against 40 gut bacterial strains, demonstrating that 24% of the drugs inhibited the growth of at least one of the bacterial strains.[108]
Effects of exercise[]
Gut microbiota and exercise have recently been shown to be interconnected. Both moderate and intense exercise are typically part of the training regimen of endurance athletes, but they exert different effects on health. The interconnection between gut microbiota and endurance sports depends upon exercise intensity and training status.[109]
Role in disease[]
Bacteria in the digestive tract can contribute to and be affected by disease in various ways. The presence or overabundance of some kinds of bacteria may contribute to inflammatory disorders such as inflammatory bowel disease.[5] Additionally, metabolites from certain members of the gut flora may influence host signalling pathways, contributing to disorders such as obesity and colon cancer.[5] Alternatively, in the event of a breakdown of the gut epithelium, the intrusion of gut flora components into other host compartments can lead to sepsis.[5]
Ulcers[]
Helicobacter pylori infection can initiate formation of stomach ulcers when the bacteria penetrate the stomach epithelial lining, then causing an inflammatory phagocytotic response.[110] In turn, the inflammation damages parietal cells which release excessive hydrochloric acid into the stomach and produce less of the protective mucus.[111] Injury to the stomach lining, leading to ulcers, develops when gastric acid overwhelms the defensive properties of cells and inhibits endogenous prostaglandin synthesis, reduces mucus and bicarbonate secretion, reduces mucosal blood flow, and lowers resistance to injury.[111] Reduced protective properties of the stomach lining increase vulnerability to further injury and ulcer formation by stomach acid, pepsin, and bile salts.[110][111]
Bowel perforation[]
Normally-commensal bacteria can harm the host if they extrude from the intestinal tract.[9][10] Translocation, which occurs when bacteria leave the gut through its mucosal lining, can occur in a number of different diseases.[10] If the gut is perforated, bacteria invade the interstitium, causing a potentially fatal infection.[4]:715
Inflammatory bowel diseases[]
The two main types of inflammatory bowel diseases, Crohn's disease and ulcerative colitis, are chronic inflammatory disorders of the gut; the causes of these diseases are unknown and issues with the gut flora and its relationship with the host have been implicated in these conditions.[12][112][113][114] Additionally, it appears that interactions of gut flora with the gut-brain axis have a role in IBD, with physiological stress mediated through the hypothalamic–pituitary–adrenal axis driving changes to intestinal epithelium and the gut flora in turn releasing factors and metabolites that trigger signaling in the enteric nervous system and the vagus nerve.[3]
The diversity of gut flora appears to be significantly diminished in people with inflammatory bowel diseases compared to healthy people; additionally, in people with ulcerative colitis, Proteobacteria and Actinobacteria appear to dominate; in people with Crohn's, Enterococcus faecium and several Proteobacteria appear to be over-represented.[3]
There is reasonable evidence that correcting gut flora imbalances by taking probiotics with Lactobacilli and Bifidobacteria can reduce visceral pain and gut inflammation in IBD.[96]
Irritable bowel syndrome[]
Irritable bowel syndrome is a result of stress and chronic activation of the HPA axis; its symptoms include abdominal pain, changes in bowel movements, and an increase in proinflammatory cytokines. Overall, studies have found that the luminal and mucosal microbiota are changed in irritable bowel syndrome individuals, and these changes can relate to the type of irritation such as diarrhea or constipation. Also, there is a decrease in the diversity of the microbiome with low levels of fecal Lactobacilli and Bifidobacteria, high levels of facultative anaerobic bacteria such as Escherichia coli, and increased ratios of Firmicutes: Bacteroidetes.[90]
Other inflammatory or autoimmune conditions[]
Allergy, asthma, and diabetes mellitus are autoimmune and inflammatory disorders of unknown cause, but have been linked to imbalances in the gut flora and its relationship with the host.[12] As of 2016 it was not clear if changes to the gut flora cause these auto-immune and inflammatory disorders or are a product of or adaptation to them.[12][115]
Asthma[]
With asthma, two hypotheses have been posed to explain its rising prevalence in the developed world. The hygiene hypothesis posits that children in the developed world are not exposed to enough microbes and thus may contain lower prevalence of specific bacterial taxa that play protective roles.[116] The second hypothesis focuses on the Western pattern diet, which lacks whole grains and fiber and has an overabundance of simple sugars.[12] Both hypotheses converge on the role of short-chain fatty acids (SCFAs) in immunomodulation. These bacterial fermentation metabolites are involved in immune signalling that prevents the triggering of asthma and lower SCFA levels are associated with the disease.[116][117] Lacking protective genera such as Lachnospira, Veillonella, Rothia and Faecalibacterium has been linked to reduced SCFA levels.[116] Further, SCFAs are the product of bacterial fermentation of fiber, which is low in the Western pattern diet.[12][117] SCFAs offer a link between gut flora and immune disorders, and as of 2016, this was an active area of research.[12] Similar hypotheses have also been posited for the rise of food and other allergies.[118]
Diabetes mellitus type 1[]
The connection between the gut microbiota and diabetes mellitus type 1 has also been linked to SCFAs, such as butyrate and acetate. Diets yielding butyrate and acetate from bacterial fermentation show increased Treg expression.[119] Treg cells downregulate effector T cells, which in turn reduces the inflammatory response in the gut.[120] Butyrate is an energy source for colon cells. butyrate-yielding diets thus decrease gut permeability by providing sufficient energy for the formation of tight junctions.[121] Additionally, butyrate has also been shown to decrease insulin resistance, suggesting gut communities low in butyrate-producing microbes may increase chances of acquiring diabetes mellitus type 2.[122] Butyrate-yielding diets may also have potential colorectal cancer suppression effects.[121]
Obesity and metabolic syndrome[]
The gut flora has also been implicated in obesity and metabolic syndrome due to the key role it plays in the digestive process; the Western pattern diet appears to drive and maintain changes in the gut flora that in turn change how much energy is derived from food and how that energy is used.[114][123] One aspect of a healthy diet that is often lacking in the Western-pattern diet is fiber and other complex carbohydrates that a healthy gut flora require flourishing; changes to gut flora in response to a Western-pattern diet appear to increase the amount of energy generated by the gut flora which may contribute to obesity and metabolic syndrome.[96] There is also evidence that microbiota influence eating behaviours based on the preferences of the microbiota, which can lead to the host consuming more food eventually resulting in obesity. It has generally been observed that with higher gut microbiome diversity, the microbiota will spend energy and resources on competing with other microbiota and less on manipulating the host. The opposite is seen with lower gut microbiome diversity, and these microbiotas may work together to create host food cravings.[42]
Additionally, the liver plays a dominant role in blood glucose homeostasis by maintaining a balance between the uptake and storage of glucose through the metabolic pathways of glycogenesis and gluconeogenesis. Intestinal lipids regulate glucose homeostasis involving a gut-brain-liver axis. The direct administration of lipids into the upper intestine increases the long chain fatty acyl-coenzyme A (LCFA-CoA) levels in the upper intestines and suppresses glucose production even under subdiaphragmatic vagotomy or gut vagal deafferentation. This interrupts the neural connection between the brain and the gut and blocks the upper intestinal lipids' ability to inhibit glucose production. The gut-brain-liver axis and gut microbiota composition can regulate the glucose homeostasis in the liver and provide potential therapeutic methods to treat obesity and diabetes.[124]
Just as gut flora can function in a feedback loop that can drive the development of obesity, there is evidence that restricting intake of calories (i.e., dieting) can drive changes to the composition of the gut flora.[114]
Liver disease[]
As the liver is fed directly by the portal vein, whatever crosses the intestinal epithelium and the intestinal mucosal barrier enters the liver, as do cytokines generated there.[125] Dysbiosis in the gut flora has been linked with the development of cirrhosis and non-alcoholic fatty liver disease.[125]
Cancer[]
Some genera of bacteria, such as Bacteroides and Clostridium, have been associated with an increase in tumor growth rate, while other genera, such as Lactobacillus and Bifidobacteria, are known to prevent tumor formation.[5] As of December 2017 there was preliminary and indirect evidence that gut microbiota might mediate response to PD-1 inhibitors; the mechanism was unknown.[126]
Neuropsychiatric[]
Interest in the relationship between gut flora and neuropsychiatric issues was sparked by a 2014 study showing that germ-free mice showed an exaggerated HPA axis response to stress compared to non-GF laboratory mice.[68] As of January 2016, most of the work that has been done on the role of gut flora in the gut-brain axis had been conducted in animals, or characterizing the various neuroactive compounds that gut flora can produce, and studies with humans measuring differences between people with various psychiatric and neurological differences, or changes to gut flora in response to stress, or measuring effects of various probiotics (dubbed "psychobiotics in this context), had generally been small and could not be generalized; whether changes to gut flora are a result of disease, a cause of disease, or both in any number of possible feedback loops in the gut-brain axis, remained unclear.[68][96]
A systematic review from 2016 examined the preclinical and small human trials that have been conducted with certain commercially available strains of probiotic bacteria and found that among those tested, the genera Bifidobacterium and Lactobacillus (B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei) had the most potential to be useful for certain central nervous system disorders.[13]
Other animals[]
The composition of the human gut microbiome is similar to that of the other great apes. However, humans’ gut biota has decreased in diversity and changed in composition since our evolutionary split from Pan.[127] Humans display increases in Bacteroidetes, a bacterial phylum associated with diets high in animal protein and fat, and decreases in Methanobrevibacter and Fibrobacter, groups that ferment complex plant polysaccharides.[127] These changes are the result of the combined dietary, genetic, and cultural changes humans have undergone since evolutionary divergence from Pan.
In addition to humans and vertebrates, some insects also possess complex and diverse gut microbiota that play key nutritional roles.[128] Microbial communities associated with termites can constitute a majority of the weight of the individuals and perform important roles in the digestion of lignocellulose and nitrogen fixation.[129] These communities are host-specific, and closely related insect species share comparable similarities in gut microbiota composition.[130][131] In cockroaches, gut microbiota have been shown to assemble in a deterministic fashion, irrespective of the inoculum;[132] the reason for this host-specific assembly remains unclear. Bacterial communities associated with insects like termites and cockroaches are determined by a combination of forces, primarily diet, but there is some indication that host phylogeny may also be playing a role in the selection of lineages.[130][131]
For more than 51 years it has been known that the administration of low doses of antibacterial agents promotes the growth of farm animals to increase weight gain.[95]
In a study carried out on mice the ratio of Firmicutes and Lachnospiraceae was significantly elevated in animals treated with subtherapeutic doses of different antibiotics. By analyzing the caloric content of faeces and the concentration of small chain fatty acids (SCFAs) in the GI tract, it was concluded that the changes in the composition of microbiota lead to an increased capacity to extract calories from otherwise indigestible constituents, and to an increased production of SCFAs. These findings provide evidence that antibiotics perturb not only the composition of the GI microbiome but also its metabolic capabilities, specifically with respect to SCFAs.[95]
See also[]
- Colonisation resistance
- List of human flora
- List of microbiota species of the lower reproductive tract of women
- Skin flora
- Verotoxin-producing Escherichia coli
Notes[]
References[]
- ^ Moszak, M; Szulińska, M; Bogdański, P (15 April 2020). "You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review". Nutrients. 12 (4): 1096. doi:10.3390/nu12041096. PMC 7230850. PMID 32326604. S2CID 216108564.
- ^ Segata, N; Boernigen, D; Tickle, TL; Morgan, XC; Garrett, WS; Huttenhower, C (14 May 2013). "Computational meta'omics for microbial community studies". Molecular Systems Biology. 9: 666. doi:10.1038/msb.2013.22. PMC 4039370. PMID 23670539.
- ^ Jump up to: a b c Saxena, R.; Sharma, V.K (2016). "A Metagenomic Insight Into the Human Microbiome: Its Implications in Health and Disease". In D. Kumar; S. Antonarakis (eds.). Medical and Health Genomics. Elsevier Science. p. 117. doi:10.1016/B978-0-12-420196-5.00009-5. ISBN 978-0-12-799922-7.
- ^ Jump up to: a b c d e f g h i j k l m Sherwood, Linda; Willey, Joanne; Woolverton, Christopher (2013). Prescott's Microbiology (9th ed.). New York: McGraw Hill. pp. 713–21. ISBN 9780073402406. OCLC 886600661.
- ^ Jump up to: a b c d e f g h i j k l m n o p q Guarner, F; Malagelada, J (2003). "Gut flora in health and disease". The Lancet. 361 (9356): 512–19. doi:10.1016/S0140-6736(03)12489-0. PMID 12583961. S2CID 38767655.
- ^ Jump up to: a b c d e f g h i j k Beaugerie, Laurent; Petit, Jean-Claude (2004). "Antibiotic-associated diarrhoea". Best Practice & Research Clinical Gastroenterology. 18 (2): 337–52. doi:10.1016/j.bpg.2003.10.002. PMID 15123074.
- ^ Jump up to: a b Stephen, A. M.; Cummings, J. H. (1980). "The Microbial Contribution to Human Faecal Mass". Journal of Medical Microbiology. 13 (1): 45–56. doi:10.1099/00222615-13-1-45. PMID 7359576.
- ^ Jump up to: a b c d e Quigley, E. M (2013). "Gut bacteria in health and disease". Gastroenterology & Hepatology. 9 (9): 560–9. PMC 3983973. PMID 24729765.
- ^ Jump up to: a b c d e f g h i j k l m n o p q Sommer, Felix; Bäckhed, Fredrik (2013). "The gut microbiota — masters of host development and physiology". Nature Reviews Microbiology. 11 (4): 227–38. doi:10.1038/nrmicro2974. PMID 23435359. S2CID 22798964.
- ^ Jump up to: a b c d Faderl, Martin; Noti, Mario; Corazza, Nadia; Mueller, Christoph (2015). "Keeping bugs in check: The mucus layer as a critical component in maintaining intestinal homeostasis". IUBMB Life. 67 (4): 275–85. doi:10.1002/iub.1374. PMID 25914114. S2CID 25878594.
- ^ Jump up to: a b c d e f Clarke, Gerard; Stilling, Roman M; Kennedy, Paul J; Stanton, Catherine; Cryan, John F; Dinan, Timothy G (2014). "Minireview: Gut Microbiota: The Neglected Endocrine Organ". Molecular Endocrinology. 28 (8): 1221–38. doi:10.1210/me.2014-1108. PMC 5414803. PMID 24892638.
- ^ Jump up to: a b c d e f g h Shen, Sj; Wong, Connie HY (2016). "Bugging inflammation: Role of the gut microbiota". Clinical & Translational Immunology. 5 (4): e72. doi:10.1038/cti.2016.12. PMC 4855262. PMID 27195115.
- ^ Jump up to: a b c Wang, Huiying; Lee, In-Seon; Braun, Christoph; Enck, Paul (2016). "Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review". Journal of Neurogastroenterology and Motility. 22 (4): 589–605. doi:10.5056/jnm16018. PMC 5056568. PMID 27413138.
- ^ Jump up to: a b c d e f Sears, Cynthia L. (2005). "A dynamic partnership: Celebrating our gut flora". Anaerobe. 11 (5): 247–51. doi:10.1016/j.anaerobe.2005.05.001. PMID 16701579.
- ^ Shapira, Michael (2016-07-01). "Gut Microbiotas and Host Evolution: Scaling Up Symbiosis". Trends in Ecology & Evolution. 31 (7): 539–549. doi:10.1016/j.tree.2016.03.006. ISSN 0169-5347. PMID 27039196.
- ^ Lozupone, Catherine A.; Stombaugh, Jesse I.; Gordon, Jeffrey I.; Jansson, Janet K.; Knight, Rob (2012). "Diversity, stability and resilience of the human gut microbiota". Nature. 489 (7415): 220–30. Bibcode:2012Natur.489..220L. doi:10.1038/nature11550. PMC 3577372. PMID 22972295.
- ^ Qin, Junjie; Li, Ruiqiang; Raes, Jeroen; Arumugam, Manimozhiyan; Burgdorf, Kristoffer Solvsten; Manichanh, Chaysavanh; Nielsen, Trine; Pons, Nicolas; Levenez, Florence; Yamada, Takuji; Mende, Daniel R.; Li, Junhua; Xu, Junming; Li, Shaochuan; Li, Dongfang; Cao, Jianjun; Wang, Bo; Liang, Huiqing; Zheng, Huisong; Xie, Yinlong; Tap, Julien; Lepage, Patricia; Bertalan, Marcelo; Batto, Jean-Michel; Hansen, Torben; Le Paslier, Denis; Linneberg, Allan; Nielsen, H. Bjørn; Pelletier, Eric; Renault, Pierre (2010). "A human gut microbial gene catalogue established by metagenomic sequencing". Nature. 464 (7285): 59–65. Bibcode:2010Natur.464...59.. doi:10.1038/nature08821. PMC 3779803. PMID 20203603.
- ^ Shanahan, Fergus (2002). "The host–microbe interface within the gut". Best Practice & Research Clinical Gastroenterology. 16 (6): 915–31. doi:10.1053/bega.2002.0342. PMID 12473298.
- ^ Tap, Julien; Mondot, Stanislas; Levenez, Florence; Pelletier, Eric; Caron, Christophe; Furet, Jean-Pierre; Ugarte, Edgardo; Muñoz-Tamayo, Rafael; Paslier, Denis L. E.; Nalin, Renaud; Dore, Joel; Leclerc, Marion (2009). "Towards the human intestinal microbiota phylogenetic core". Environmental Microbiology. 11 (10): 2574–84. doi:10.1111/j.1462-2920.2009.01982.x. PMID 19601958.
- ^ Jump up to: a b O'Hara, Ann M; Shanahan, Fergus (2006). "The gut flora as a forgotten organ". EMBO Reports. 7 (7): 688–93. doi:10.1038/sj.embor.7400731. PMC 1500832. PMID 16819463.
- ^ Khanna, Sahil; Tosh, Pritish K (2014). "A Clinician's Primer on the Role of the Microbiome in Human Health and Disease". Mayo Clinic Proceedings. 89 (1): 107–14. doi:10.1016/j.mayocp.2013.10.011. PMID 24388028.
- ^ Jump up to: a b Cui, Lijia; Morris, Alison; Ghedin, Elodie (2013). "The human mycobiome in health and disease". Genome Medicine. 5 (7): 63. doi:10.1186/gm467. PMC 3978422. PMID 23899327.
- ^ Erdogan, Askin; Rao, Satish S. C (2015). "Small Intestinal Fungal Overgrowth". Current Gastroenterology Reports. 17 (4): 16. doi:10.1007/s11894-015-0436-2. PMID 25786900. S2CID 3098136.
- ^ Bello, Maria G. Dominguez; Knight, Rob; Gilbert, Jack A.; Blaser, Martin J. (4 October 2018). "Preserving microbial diversity". Science. 362 (6410): 33–34. Bibcode:2018Sci...362...33B. doi:10.1126/science.aau8816. PMID 30287652. S2CID 52919917.
- ^ Jump up to: a b Arumugam, Manimozhiyan; Raes, Jeroen; Pelletier, Eric; Le Paslier, Denis; Yamada, Takuji; Mende, Daniel R.; Fernandes, Gabriel R.; Tap, Julien; Bruls, Thomas; Batto, Jean-Michel; Bertalan, Marcelo; Borruel, Natalia; Casellas, Francesc; Fernandez, Leyden; Gautier, Laurent; Hansen, Torben; Hattori, Masahira; Hayashi, Tetsuya; Kleerebezem, Michiel; Kurokawa, Ken; Leclerc, Marion; Levenez, Florence; Manichanh, Chaysavanh; Nielsen, H. Bjørn; Nielsen, Trine; Pons, Nicolas; Poulain, Julie; Qin, Junjie; Sicheritz-Ponten, Thomas; Tims, Sebastian (2011). "Enterotypes of the human gut microbiome". Nature. 473 (7346): 174–80. Bibcode:2011Natur.473..174.. doi:10.1038/nature09944. PMC 3728647. PMID 21508958.
- ^ Jump up to: a b Wu, G. D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S. A.; Bewtra, M.; Knights, D.; Walters, W. A.; Knight, R.; Sinha, R.; Gilroy, E.; Gupta, K.; Baldassano, R.; Nessel, L.; Li, H.; Bushman, F. D.; Lewis, J. D. (2011). "Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes". Science. 334 (6052): 105–08. Bibcode:2011Sci...334..105W. doi:10.1126/science.1208344. PMC 3368382. PMID 21885731.
- ^ Zimmer, Carl (April 20, 2011). "Bacteria Divide People Into 3 Types, Scientists Say". The New York Times. Retrieved April 21, 2011.
a group of scientists now report just three distinct ecosystems in the guts of people they have studied.
- ^ Knights, Dan; Ward, Tonya; McKinlay, Christopher; Miller, Hannah; Gonzalez, Antonio; McDonald, Daniel; Knight, Rob (8 October 2014). "Rethinking "Enterotypes"". Cell Host & Microbe. 16 (4): 433–37. doi:10.1016/j.chom.2014.09.013. PMC 5558460. PMID 25299329.
- ^ Kenneth Todar (2012). "The Normal Bacterial Flora of Humans". Todar's Online Textbook of Bacteriology. Retrieved June 25, 2016.
- ^ Quigley, Eamonn M.M; Quera, Rodrigo (2006). "Small Intestinal Bacterial Overgrowth: Roles of Antibiotics, Prebiotics, and Probiotics". Gastroenterology. 130 (2): S78–90. doi:10.1053/j.gastro.2005.11.046. PMID 16473077. S2CID 16904501.
- ^ Adams, M. R.; Moss, M. O. (2007). Food Microbiology. doi:10.1039/9781847557940. ISBN 978-0-85404-284-5.
- ^ Jump up to: a b University of Glasgow. 2005. The normal gut flora. Available through web archive. Accessed May 22, 2008
- ^ Braune A, Blaut M (2016). "Bacterial species involved in the conversion of dietary flavonoids in the human gut". Gut Microbes. 7 (3): 216–234. doi:10.1080/19490976.2016.1158395. PMC 4939924. PMID 26963713.
- ^ Jump up to: a b Steinhoff, U (2005). "Who controls the crowd? New findings and old questions about the intestinal microflora". Immunology Letters. 99 (1): 12–16. doi:10.1016/j.imlet.2004.12.013. PMID 15894105.
- ^ Jump up to: a b c d e f Gibson, Glenn R (2004). "Fibre and effects on probiotics (the prebiotic concept)". Clinical Nutrition Supplements. 1 (2): 25–31. doi:10.1016/j.clnu.2004.09.005.
- ^ Miquel, S; Martín, R; Rossi, O; Bermúdez-Humarán, LG; Chatel, JM; Sokol, H; Thomas, M; Wells, JM; Langella, P (2013). "Faecalibacterium prausnitzii and human intestinal health". Current Opinion in Microbiology. 16 (3): 255–61. doi:10.1016/j.mib.2013.06.003. PMID 23831042.
- ^ Ley, Ruth E (2010). "Obesity and the human microbiome". Current Opinion in Gastroenterology. 26 (1): 5–11. doi:10.1097/MOG.0b013e328333d751. PMID 19901833. S2CID 23329156.
- ^ Nash, Andrea K; Auchtung, Thomas A; Wong, Matthew C; Smith, Daniel P; Gesell, Jonathan R; Ross, Matthew C; Stewart, Christopher J; Metcalf, Ginger A; Muzny, Donna M; Gibbs, Richard A; Ajami, Nadim J; Petrosino, Joseph F (2017). "The gut mycobiome of the Human Microbiome Project healthy cohort". Microbiome. 5 (1): 153. doi:10.1186/s40168-017-0373-4. PMC 5702186. PMID 29178920.
- ^ Scarpellini, Emidio; Ianiro, Gianluca; Attili, Fabia; Bassanelli, Chiara; De Santis, Adriano; Gasbarrini, Antonio (2015). "The human gut microbiota and virome: Potential therapeutic implications". Digestive and Liver Disease. 47 (12): 1007–12. doi:10.1016/j.dld.2015.07.008. PMC 7185617. PMID 26257129.
- ^ Gerritsen, Jacoline; Smidt, Hauke; Rijkers, Ger; de Vos, Willem (27 May 2011). "Intestinal microbiota in human health and disease: the impact of probiotics". Genes & Nutrition. 6 (3): 209–40. doi:10.1007/s12263-011-0229-7. PMC 3145058. PMID 21617937.
- ^ Jump up to: a b c d e f Yatsunenko, T.; Rey, F. E.; Manary, M. J.; Trehan, I.; Dominguez-Bello, M. G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R. N.; Anokhin, A. P.; Heath, A. C.; Warner, B.; Reeder, J.; Kuczynski, J.; Caporaso, J. G.; Lozupone, C. A.; Lauber, C.; Clemente, J. C.; Knights, D.; Knight, R.; Gordon, J. I. (2012). "Human gut microbiome viewed across age and geography". Nature. 486 (7402): 222–27. Bibcode:2012Natur.486..222Y. doi:10.1038/nature11053. PMC 3376388. PMID 22699611.
- ^ Jump up to: a b c Alcock, Joe; Maley, Carlo C; Aktipis, C. Athena (2014). "Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms". BioEssays. 36 (10): 940–9. doi:10.1002/bies.201400071. PMC 4270213. PMID 25103109.
- ^ "Study finds childhood diet has lifelong impact". phys.org. Retrieved 13 February 2021.
- ^ "Poor diet in childhood may have lasting effects on gut microbiome". New Atlas. 4 February 2021. Retrieved 13 February 2021.
- ^ McNamara, Monica P.; Singleton, Jennifer M.; Cadney, Marcell D.; Ruegger, Paul M.; Borneman, James; Garland, Theodore (1 January 2021). "Early-life effects of juvenile Western diet and exercise on adult gut microbiome composition in mice". Journal of Experimental Biology. 224 (4): jeb.239699. doi:10.1242/jeb.239699. ISSN 0022-0949. PMC 7929929. PMID 33431595.
- ^ Yeh, Ming-Chin; Glick-Bauer, Marian (November 2014). "The Health Advantage of a Vegan Diet: Exploring the Gut Microbiota Connection". Nutrients. 6 (11): 4822–4838. doi:10.3390/nu6114822. PMC 4245565. PMID 25365383.
- ^ Jump up to: a b David, Lawrence A.; Maurice, Corinne F.; Carmody, Rachel N.; Gootenberg, David B.; Button, Julie E.; Wolfe, Benjamin E.; Ling, Alisha V.; Devlin, A. Sloan; Varma, Yug; Fischbach, Michael A.; Biddinger, Sudha B.; Dutton, Rachel J.; Turnbaugh, Peter J. (11 December 2013). "Diet rapidly and reproducibly alters the human gut microbiome". Nature. 505 (7484): 559–563. doi:10.1038/nature12820. PMC 3957428. PMID 24336217.
- ^ Jeffery, Ian; O'Toole, Paul (17 January 2013). "Diet-Microbiota Interactions and Their Implications for Healthy Living". Nutrients. 5 (1): 234–252. doi:10.3390/nu5010234. PMC 3571646. PMID 23344252.
- ^ De Filippo, C; Cavalieri, D; Di Paola, M; Ramazzotti, M; Poullet, J. B; Massart, S; Collini, S; Pieraccini, G; Lionetti, P (2010). "Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa". Proceedings of the National Academy of Sciences. 107 (33): 14691–6. Bibcode:2010PNAS..10714691D. doi:10.1073/pnas.1005963107. PMC 2930426. PMID 20679230.
- ^ Jonkers, Daisy M.A.E. (2016). "Microbial perturbations and modulation in conditions associated with malnutrition and malabsorption". Best Practice & Research Clinical Gastroenterology. 30 (2): 161–72. doi:10.1016/j.bpg.2016.02.006. PMID 27086883.
- ^ Million, Matthieu; Diallo, Aldiouma; Raoult, Didier (May 2017). "Gut microbiota and malnutrition". Microbial Pathogenesis. 106: 127–138. doi:10.1016/j.micpath.2016.02.003. PMID 26853753.
- ^ Rytter, Maren Johanne Heilskov; Kolte, Lilian; Briend, André; Friis, Henrik; Christensen, Vibeke Brix (2014). "The Immune System in Children with Malnutrition—A Systematic Review". PLOS ONE. 9 (8): e105017. Bibcode:2014PLoSO...9j5017R. doi:10.1371/journal.pone.0105017. PMC 4143239. PMID 25153531.
- ^ Jump up to: a b c Renson, Audrey; Herd, Pamela; Dowd, Jennifer B. (2020). "Sick Individuals and Sick (Microbial) Populations: Challenges in Epidemiology and the Microbiome". Annual Review of Public Health. 41: 63–80. doi:10.1146/annurev-publhealth-040119-094423. PMID 31635533.
- ^ Turroni, Francesca; Peano, Clelia; Pass, Daniel A; Foroni, Elena; Severgnini, Marco; Claesson, Marcus J; Kerr, Colm; Hourihane, Jonathan; Murray, Deirdre; Fuligni, Fabio; Gueimonde, Miguel; Margolles, Abelardo; De Bellis, Gianluca; o'Toole, Paul W; Van Sinderen, Douwe; Marchesi, Julian R; Ventura, Marco (2012). "Diversity of Bifidobacteria within the Infant Gut Microbiota". PLOS ONE. 7 (5): e36957. Bibcode:2012PLoSO...736957T. doi:10.1371/journal.pone.0036957. PMC 3350489. PMID 22606315.
- ^ Davenport, Emily R.; Sanders, Jon G.; Song, Se Jin; Amato, Katherine R.; Clark, Andrew G.; Knight, Rob (2017-12-27). "The human microbiome in evolution". BMC Biology. 15 (1): 127. doi:10.1186/s12915-017-0454-7. ISSN 1741-7007. PMC 5744394. PMID 29282061.
- ^ Perez-Muñoz, Maria Elisa; Arrieta, Marie-Claire; Ramer-Tait, Amanda E; Walter, Jens (2017). "A critical assessment of the 'sterile womb' and 'in utero colonization' hypotheses: Implications for research on the pioneer infant microbiome". Microbiome. 5 (1): 48. doi:10.1186/s40168-017-0268-4. PMC 5410102. PMID 28454555.
- ^ Jump up to: a b Matamoros, Sebastien; Gras-Leguen, Christele; Le Vacon, Françoise; Potel, Gilles; de la Cochetiere, Marie-France (2013). "Development of intestinal microbiota in infants and its impact on health". Trends in Microbiology. 21 (4): 167–73. doi:10.1016/j.tim.2012.12.001. PMID 23332725.
- ^ Jump up to: a b c d e f g Mueller, Noel T.; Bakacs, Elizabeth; Combellick, Joan; Grigoryan, Zoya; Dominguez-Bello, Maria G. (2015). "The infant microbiome development: mom matters". Trends in Molecular Medicine. 21 (2): 109–17. doi:10.1016/j.molmed.2014.12.002. PMC 4464665. PMID 25578246.
- ^ Jiménez, Esther; Fernández, Leonides; Marín, María L; Martín, Rocío; Odriozola, Juan M; Nueno-Palop, Carmen; Narbad, Arjan; Olivares, Mónica; Xaus, Jordi; Rodríguez, Juan M (2005). "Isolation of Commensal Bacteria from Umbilical Cord Blood of Healthy Neonates Born by Cesarean Section". Current Microbiology. 51 (4): 270–4. doi:10.1007/s00284-005-0020-3. PMID 16187156. S2CID 43438656.
- ^ Collado, Maria Carmen; Rautava, Samuli; Aakko, Juhani; Isolauri, Erika; Salminen, Seppo (2016). "Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid". Scientific Reports. 6: 23129. Bibcode:2016NatSR...623129C. doi:10.1038/srep23129. PMC 4802384. PMID 27001291.
- ^ Jiménez, Esther; Marín, María L.; Martín, Rocío; Odriozola, Juan M.; Olivares, Mónica; Xaus, Jordi; Fernández, Leonides; Rodríguez, Juan M. (2008). "Is meconium from healthy newborns actually sterile?". Research in Microbiology. 159 (3): 187–93. doi:10.1016/j.resmic.2007.12.007. PMID 18281199.
- ^ Perez-Muñoz, Maria Elisa; Arrieta, Marie-Claire; Ramer-Tait, Amanda E; Walter, Jens (2017). "A critical assessment of the "sterile womb" and "in utero colonization" hypotheses: Implications for research on the pioneer infant microbiome". Microbiome. 5 (1): 48. doi:10.1186/s40168-017-0268-4. PMC 5410102. PMID 28454555.
- ^ Adlerberth, I; Wold, AE (2009). "Establishment of the gut microbiota in Western infants". Acta Paediatrica. 98 (2): 229–38. doi:10.1111/j.1651-2227.2008.01060.x. PMID 19143664. S2CID 205859933.
- ^ Coppa, G.V; Zampini, L; Galeazzi, T; Gabrielli, O (2006). "Prebiotics in human milk: A review". Digestive and Liver Disease. 38: S291–4. doi:10.1016/S1590-8658(07)60013-9. PMID 17259094.
- ^ Fanaro, S; Chierici, R; Guerrini, P; Vigi, V (2007). "Intestinal microflora in early infancy: Composition and development". Acta Paediatrica. 92 (441): 48–55. doi:10.1111/j.1651-2227.2003.tb00646.x. PMID 14599042. S2CID 10316311.
- ^ Yassour, Moran; Vatanen, Tommi; Siljander, Heli; Hämäläinen, Anu-Maaria; Härkönen, Taina; Ryhänen, Samppa J; Franzosa, Eric A; Vlamakis, Hera; Huttenhower, Curtis; Gevers, Dirk; Lander, Eric S; Knip, Mikael; Xavier, Ramnik J (2016). "Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability". Science Translational Medicine. 8 (343): 343ra81. doi:10.1126/scitranslmed.aad0917. PMC 5032909. PMID 27306663.
- ^ Gibson, G. R.; Roberfroid, M. B. (1995). "Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics". The Journal of Nutrition. 125 (6): 1401–1412. doi:10.1093/jn/125.6.1401. PMID 7782892.
- ^ Jump up to: a b c d e f Wang, Yan; Kasper, Lloyd H (2014). "The role of microbiome in central nervous system disorders". Brain, Behavior, and Immunity. 38: 1–12. doi:10.1016/j.bbi.2013.12.015. PMC 4062078. PMID 24370461.
- ^ Jump up to: a b Yoon, My Young; Lee, Keehoon; Yoon, Sang Sun (2014). "Protective role of gut commensal microbes against intestinal infections". Journal of Microbiology. 52 (12): 983–9. doi:10.1007/s12275-014-4655-2. PMID 25467115. S2CID 54622675.
- ^ Reinoso Webb, Cynthia; Koboziev, Iurii; Furr, Kathryn L; Grisham, Matthew B (2016). "Protective and pro-inflammatory roles of intestinal bacteria". Pathophysiology. 23 (2): 67–80. doi:10.1016/j.pathophys.2016.02.002. PMC 4867289. PMID 26947707.
- ^ Mantis, N J; Rol, N; Corthésy, B (2011). "Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut". Mucosal Immunology. 4 (6): 603–11. doi:10.1038/mi.2011.41. PMC 3774538. PMID 21975936.
- ^ Jump up to: a b Peterson, Lance W; Artis, David (2014). "Intestinal epithelial cells: Regulators of barrier function and immune homeostasis". Nature Reviews Immunology. 14 (3): 141–53. doi:10.1038/nri3608. PMID 24566914. S2CID 3351351.
- ^ Jump up to: a b Honda, Kenya; Littman, Dan R (2016). "The microbiota in adaptive immune homeostasis and disease". Nature. 535 (7610): 75–84. Bibcode:2016Natur.535...75H. doi:10.1038/nature18848. PMID 27383982. S2CID 4461492.
- ^ Jump up to: a b Levy, M.; Thaiss, C.A.; Elinav, E. (2016). "Metabolites: messengers between the microbiota and the immune system". Genes & Development. 30 (14): 1589–97. doi:10.1101/gad.284091.116. PMC 4973288. PMID 27474437.
- ^ Jump up to: a b c d e f g h i Zhang LS, Davies SS (April 2016). "Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions". Genome Med. 8 (1): 46. doi:10.1186/s13073-016-0296-x. PMC 4840492. PMID 27102537.
Lactobacillus spp. convert tryptophan to indole-3-aldehyde (I3A) through unidentified enzymes [125]. Clostridium sporogenes convert tryptophan to IPA [6], likely via a tryptophan deaminase. ... IPA also potently scavenges hydroxyl radicals
Table 2: Microbial metabolites: their synthesis, mechanisms of action, and effects on health and disease
Figure 1: Molecular mechanisms of action of indole and its metabolites on host physiology and disease - ^ Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G (March 2009). "Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites". Proc. Natl. Acad. Sci. U.S.A. 106 (10): 3698–3703. Bibcode:2009PNAS..106.3698W. doi:10.1073/pnas.0812874106. PMC 2656143. PMID 19234110.
Production of IPA was shown to be completely dependent on the presence of gut microflora and could be established by colonization with the bacterium Clostridium sporogenes.
IPA metabolism diagram - ^ "3-Indolepropionic acid". Human Metabolome Database. University of Alberta. Retrieved 12 June 2018.
Indole-3-propionate (IPA), a deamination product of tryptophan formed by symbiotic bacteria in the gastrointestinal tract of mammals and birds. 3-Indolepropionic acid has been shown to prevent oxidative stress and death of primary neurons and neuroblastoma cells exposed to the amyloid beta-protein in the form of amyloid fibrils, one of the most prominent neuropathologic features of Alzheimer's disease. 3-Indolepropionic acid also shows a strong level of neuroprotection in two other paradigms of oxidative stress. (PMID 10419516) ... More recently it has been found that higher indole-3-propionic acid levels in serum/plasma are associated with reduced likelihood of type 2 diabetes and with higher levels of consumption of fiber-rich foods (PMID 28397877)
Origin: • Endogenous • Microbial - ^ Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA (July 1999). "Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid". J. Biol. Chem. 274 (31): 21937–21942. doi:10.1074/jbc.274.31.21937. PMID 10419516. S2CID 6630247.
[Indole-3-propionic acid (IPA)] has previously been identified in the plasma and cerebrospinal fluid of humans, but its functions are not known. ... In kinetic competition experiments using free radical-trapping agents, the capacity of IPA to scavenge hydroxyl radicals exceeded that of melatonin, an indoleamine considered to be the most potent naturally occurring scavenger of free radicals. In contrast with other antioxidants, IPA was not converted to reactive intermediates with pro-oxidant activity.
- ^ Hopper, Christopher P.; De La Cruz, Ladie Kimberly; Lyles, Kristin V.; Wareham, Lauren K.; Gilbert, Jack A.; Eichenbaum, Zehava; Magierowski, Marcin; Poole, Robert K.; Wollborn, Jakob; Wang, Binghe (2020-12-23). "Role of Carbon Monoxide in Host–Gut Microbiome Communication". Chemical Reviews. 120 (24): 13273–13311. doi:10.1021/acs.chemrev.0c00586. ISSN 0009-2665. PMID 33089988.
- ^ Rajilić-Stojanović, Mirjana; De Vos, Willem M (2014). "The first 1000 cultured species of the human gastrointestinal microbiota". FEMS Microbiology Reviews. 38 (5): 996–1047. doi:10.1111/1574-6976.12075. PMC 4262072. PMID 24861948.
- ^ Hill, M. J. (March 1997). "Intestinal flora and endogenous vitamin synthesis". European Journal of Cancer Prevention. 6 Suppl 1: S43–45. doi:10.1097/00008469-199703001-00009. ISSN 0959-8278. PMID 9167138. S2CID 8740364.
- ^ "The Microbiome". Tufts Now. 2013-09-17. Retrieved 2020-12-09.
- ^ Jump up to: a b c d e ElRakaiby M, Dutilh BE, Rizkallah MR, Boleij A, Cole JN, Aziz RK (July 2014). "Pharmacomicrobiomics: the impact of human microbiome variations on systems pharmacology and personalized therapeutics". Omics. 18 (7): 402–414. doi:10.1089/omi.2014.0018. PMC 4086029. PMID 24785449.
The hundred trillion microbes and viruses residing in every human body, which outnumber human cells and contribute at least 100 times more genes than those encoded on the human genome (Ley et al., 2006), offer an immense accessory pool for inter-individual genetic variation that has been underestimated and largely unexplored (Savage, 1977; Medini et al., 2008; Minot et al., 2011; Wylie et al., 2012). ... Meanwhile, a wealth of literature has long been available about the biotransformation of xenobiotics, notably by gut bacteria (reviewed in Sousa et al., 2008; Rizkallah et al., 2010; Johnson et al., 2012; Haiser and Turnbaugh, 2013). This valuable information is predominantly about drug metabolism by unknown human-associated microbes; however, only a few cases of inter-individual microbiome variations have been documented [e.g., digoxin (Mathan et al., 1989) and acetaminophen (Clayton et al., 2009)].
- ^ Jump up to: a b c Cho I, Blaser MJ (March 2012). "The human microbiome: at the interface of health and disease". Nature Reviews. Genetics. 13 (4): 260–270. doi:10.1038/nrg3182. PMC 3418802. PMID 22411464.
The composition of the microbiome varies by anatomical site (Figure 1). The primary determinant of community composition is anatomical location: interpersonal variation is substantial23,24 and is higher than the temporal variability seen at most sites in a single individual25. ... How does the microbiome affect the pharmacology of medications? Can we “micro-type” people to improve pharmacokinetics and/or reduce toxicity? Can we manipulate the microbiome to improve pharmacokinetic stability?
- ^ Hutter T, Gimbert C, Bouchard F, Lapointe FJ (2015). "Being human is a gut feeling". Microbiome. 3: 9. doi:10.1186/s40168-015-0076-7. PMC 4359430. PMID 25774294.
Some metagenomic studies have suggested that less than 10% of the cells that comprise our bodies are Homo sapiens cells. The remaining 90% are bacterial cells. The description of this so-called human microbiome is of great interest and importance for several reasons. For one, it helps us redefine what a biological individual is. We suggest that a human individual is now best described as a super-individual in which a large number of different species (including Homo sapiens) coexist.
- ^ Kumar K, Dhoke GV, Sharma AK, Jaiswal SK, Sharma VK (January 2019). "Mechanistic elucidation of amphetamine metabolism by tyramine oxidase from human gut microbiota using molecular dynamics simulations". Journal of Cellular Biochemistry. 120 (7): 11206–11215. doi:10.1002/jcb.28396. PMID 30701587. S2CID 73413138.
Particularly in the case of the human gut, which harbors a large diversity of bacterial species, the differences in microbial composition can significantly alter the metabolic activity in the gut lumen.4 The differential metabolic activity due to the differences in gut microbial species has been recently linked with various metabolic disorders and diseases.5-12 In addition to the impact of gut microbial diversity or dysbiosis in various human diseases, there is an increasing amount of evidence which shows that the gut microbes can affect the bioavailability and efficacy of various orally administrated drug molecules through promiscuous enzymatic metabolism.13,14 ... The present study on the atomistic details of amphetamine binding and binding affinity to the tyramine oxidase along with the comparison with two natural substrates of this enzyme namely tyramine and phenylalanine provides strong evidence for the promiscuity‐based metabolism of amphetamine by the tyramine oxidase enzyme of E. coli. The obtained results will be crucial in designing a surrogate molecule for amphetamine that can help either in improving the efficacy and bioavailability of the amphetamine drug via competitive inhibition or in redesigning the drug for better pharmacological effects. This study will also have useful clinical implications in reducing the gut microbiota caused variation in the drug response among different populations.
- ^ Sousa, Tiago; Paterson, Ronnie; Moore, Vanessa; Carlsson, Anders; Abrahamsson, Bertil; Basit, Abdul W (2008). "The gastrointestinal microbiota as a site for the biotransformation of drugs". International Journal of Pharmaceutics. 363 (1–2): 1–25. doi:10.1016/j.ijpharm.2008.07.009. PMID 18682282.
- ^ Haiser, H. J; Gootenberg, D. B; Chatman, K; Sirasani, G; Balskus, E. P; Turnbaugh, P. J (2013). "Predicting and Manipulating Cardiac Drug Inactivation by the Human Gut Bacterium Eggerthella lenta". Science. 341 (6143): 295–8. Bibcode:2013Sci...341..295H. doi:10.1126/science.1235872. PMC 3736355. PMID 23869020.
- ^ Mayer, E. A; Knight, R; Mazmanian, S. K; Cryan, J. F; Tillisch, K (2014). "Gut Microbes and the Brain: Paradigm Shift in Neuroscience". Journal of Neuroscience. 34 (46): 15490–6. doi:10.1523/JNEUROSCI.3299-14.2014. PMC 4228144. PMID 25392516.
- ^ Jump up to: a b c Dinan, Timothy G; Cryan, John F (2015). "The impact of gut microbiota on brain and behaviour". Current Opinion in Clinical Nutrition and Metabolic Care. 18 (6): 552–8. doi:10.1097/MCO.0000000000000221. PMID 26372511. S2CID 21424690.
- ^ Jump up to: a b c Carman, Robert J.; Simon, Mary Alice; Fernández, Haydée; Miller, Margaret A.; Bartholomew, Mary J. (2004). "Ciprofloxacin at low levels disrupts colonization resistance of human fecal microflora growing in chemostats". Regulatory Toxicology and Pharmacology. 40 (3): 319–26. doi:10.1016/j.yrtph.2004.08.005. PMID 15546686.
- ^ Hvas, Christian Lodberg; Baunwall, Simon Mark Dahl; Erikstrup, Christian (2020-07-01). "Faecal microbiota transplantation: A life-saving therapy challenged by commercial claims for exclusivity". EClinicalMedicine. 24: 100436. doi:10.1016/j.eclinm.2020.100436. ISSN 2589-5370. PMC 7334803. PMID 32642633.
- ^ Brandt, Lawrence J.; Borody, Thomas Julius; Campbell, Jordana (2011). "Endoscopic Fecal Microbiota Transplantation". Journal of Clinical Gastroenterology. 45 (8): 655–57. doi:10.1097/MCG.0b013e3182257d4f. PMID 21716124.
- ^ Knight, DJW; Girling, KJ (2003). "Gut flora in health and disease". The Lancet. 361 (9371): 512–19. doi:10.1016/S0140-6736(03)13438-1. PMID 12781578. S2CID 40683723.
- ^ Jump up to: a b c Cho, I.; Yamanishi, S.; Cox, L.; Methé, B. A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; Li, H.; Alekseyenko, A. V.; Blaser, M. J. (2012). "Antibiotics in early life alter the murine colonic microbiome and adiposity". Nature. 488 (7413): 621–26. Bibcode:2012Natur.488..621C. doi:10.1038/nature11400. PMC 3553221. PMID 22914093.
- ^ Jump up to: a b c d Schneiderhan, J; Master-Hunter, T; Locke, A (2016). "Targeting gut flora to treat and prevent disease". The Journal of Family Practice. 65 (1): 34–8. PMID 26845162.
- ^ Baker, Monya (2012). "Pregnancy alters resident gut microbes". Nature. doi:10.1038/nature.2012.11118. S2CID 87078157.
- ^ Hill, Colin; Guarner, Francisco; Reid, Gregor; Gibson, Glenn R; Merenstein, Daniel J; Pot, Bruno; Morelli, Lorenzo; Canani, Roberto Berni; Flint, Harry J; Salminen, Seppo; Calder, Philip C; Sanders, Mary Ellen (2014). "The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic". Nature Reviews Gastroenterology & Hepatology. 11 (8): 506–14. doi:10.1038/nrgastro.2014.66. PMID 24912386.
- ^ Rijkers, Ger T; De Vos, Willem M; Brummer, Robert-Jan; Morelli, Lorenzo; Corthier, Gerard; Marteau, Philippe (2011). "Health benefits and health claims of probiotics: Bridging science and marketing". British Journal of Nutrition. 106 (9): 1291–6. doi:10.1017/S000711451100287X. PMID 21861940.
- ^ Hutkins, Robert W; Krumbeck, Janina A; Bindels, Laure B; Cani, Patrice D; Fahey, George; Goh, Yong Jun; Hamaker, Bruce; Martens, Eric C; Mills, David A; Rastal, Robert A; Vaughan, Elaine; Sanders, Mary Ellen (2016). "Prebiotics: Why definitions matter". Current Opinion in Biotechnology. 37: 1–7. doi:10.1016/j.copbio.2015.09.001. PMC 4744122. PMID 26431716.
- ^ Pandey, Kavita. R; Naik, Suresh. R; Vakil, Babu. V (2015). "Probiotics, prebiotics and synbiotics- a review". Journal of Food Science and Technology. 52 (12): 7577–87. doi:10.1007/s13197-015-1921-1. PMC 4648921. PMID 26604335.
- ^ Broeckx, Géraldine; Vandenheuvel, Dieter; Claes, Ingmar J.J; Lebeer, Sarah; Kiekens, Filip (2016). "Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics" (PDF). International Journal of Pharmaceutics. 505 (1–2): 303–18. doi:10.1016/j.ijpharm.2016.04.002. hdl:10067/1328840151162165141. PMID 27050865.
- ^ Sleator, Roy D; Hill, Colin (2009). "Rational Design of Improved Pharmabiotics". Journal of Biomedicine and Biotechnology. 2009: 275287. doi:10.1155/2009/275287. PMC 2742647. PMID 19753318.
- ^ Patterson, Elaine; Cryan, John F; Fitzgerald, Gerald F; Ross, R. Paul; Dinan, Timothy G; Stanton, Catherine (2014). "Gut microbiota, the pharmabiotics they produce and host health". Proceedings of the Nutrition Society. 73 (4): 477–89. doi:10.1017/S0029665114001426. PMID 25196939.
- ^ Ford, Alexander C; Quigley, Eamonn M M; Lacy, Brian E; Lembo, Anthony J; Saito, Yuri A; Schiller, Lawrence R; Soffer, Edy E; Spiegel, Brennan M R; Moayyedi, Paul (2014). "Efficacy of Prebiotics, Probiotics and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-analysis". The American Journal of Gastroenterology. 109 (10): 1547–61, quiz 1546, 1562. doi:10.1038/ajg.2014.202. PMID 25070051. S2CID 205100508.
- ^ Dupont, Andrew; Richards; Jelinek, Katherine A; Krill, Joseph; Rahimi, Erik; Ghouri, Yezaz (2014). "Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease". Clinical and Experimental Gastroenterology. 7: 473–87. doi:10.2147/CEG.S27530. PMC 4266241. PMID 25525379.
- ^ Yu, Cheng Gong; Huang, Qin (2013). "Recent progress on the role of gut microbiota in the pathogenesis of inflammatory bowel disease". Journal of Digestive Diseases. 14 (10): 513–7. doi:10.1111/1751-2980.12087. PMID 23848393. S2CID 26982085.
- ^ Maier, Lisa; Pruteanu, Mihaela; Kuhn, Michael; Zeller, Georg; Telzerow, Anja; Anderson, Exene Erin; Brochado, Ana Rita; Fernandez, Keith Conrad; Dose, Hitomi; Mori, Hirotada; Patil, Kiran Raosaheb; Bork, Peer; Typas, Athanasios (2018). "Extensive impact of non-antibiotic drugs on human gut bacteria". Nature. 555 (7698): 623–628. Bibcode:2018Natur.555..623M. doi:10.1038/nature25979. PMC 6108420. PMID 29555994.
- ^ Clauss, Matthieu; Gérard, Philippe; Mosca, Alexis; Leclerc, Marion (2021). "Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance". Frontiers in Nutrition. 8: 637010. doi:10.3389/fnut.2021.637010. PMC 8222532. PMID 34179053.
- ^ Jump up to: a b Kamboj, AK; Cotter, TG; Oxentenko, AS (2017). "Helicobacter pylori: The Past, Present, and Future in Management". Mayo Clinic Proceedings. 92 (4): 599–604. doi:10.1016/j.mayocp.2016.11.017. ISSN 0025-6196. PMID 28209367.
- ^ Jump up to: a b c "Peptic ulcer disease" (PDF). The Johns Hopkins University School of Medicine. 2013. Retrieved 21 October 2020.
- ^ Burisch, Johan; Jess, Tine; Martinato, Matteo; Lakatos, Peter L (2013). "The burden of inflammatory bowel disease in Europe". Journal of Crohn's and Colitis. 7 (4): 322–37. doi:10.1016/j.crohns.2013.01.010. PMID 23395397.
- ^ Blandino, G; Inturri, R; Lazzara, F; Di Rosa, M; Malaguarnera, L (2016). "Impact of gut microbiota on diabetes mellitus". Diabetes & Metabolism. 42 (5): 303–315. doi:10.1016/j.diabet.2016.04.004. PMID 27179626.
- ^ Jump up to: a b c Boulangé, Claire L; Neves, Ana Luisa; Chilloux, Julien; Nicholson, Jeremy K; Dumas, Marc-Emmanuel (2016). "Impact of the gut microbiota on inflammation, obesity, and metabolic disease". Genome Medicine. 8 (1): 42. doi:10.1186/s13073-016-0303-2. PMC 4839080. PMID 27098727.
- ^ Spiller, Robin (2016). "Irritable bowel syndrome: New insights into symptom mechanisms and advances in treatment". F1000Research. 5: 780. doi:10.12688/f1000research.7992.1. PMC 4856111. PMID 27158477.
- ^ Jump up to: a b c Arrieta, Marie-Claire; Stiemsma, Leah T; Dimitriu, Pedro A; Thorson, Lisa; Russell, Shannon; Yurist-Doutsch, Sophie; Kuzeljevic, Boris; Gold, Matthew J; Britton, Heidi M; Lefebvre, Diana L; Subbarao, Padmaja; Mandhane, Piush; Becker, Allan; McNagny, Kelly M; Sears, Malcolm R; Kollmann, Tobias; Mohn, William W; Turvey, Stuart E; Brett Finlay, B (2015). "Early infancy microbial and metabolic alterations affect risk of childhood asthma". Science Translational Medicine. 7 (307): 307ra152. doi:10.1126/scitranslmed.aab2271. PMID 26424567. S2CID 206687974.
- ^ Jump up to: a b Stiemsma, Leah T; Turvey, Stuart E (2017). "Asthma and the microbiome: Defining the critical window in early life". Allergy, Asthma & Clinical Immunology. 13: 3. doi:10.1186/s13223-016-0173-6. PMC 5217603. PMID 28077947.
- ^ Ipci, Kagan; Altıntoprak, Niyazi; Muluk, Nuray Bayar; Senturk, Mehmet; Cingi, Cemal (2016). "The possible mechanisms of the human microbiome in allergic diseases". European Archives of Oto-Rhino-Laryngology. 274 (2): 617–626. doi:10.1007/s00405-016-4058-6. PMID 27115907. S2CID 27328940.
- ^ Mariño, E., Richards, J. L., McLeod, K. H., Stanley, D., Yap, Y. A., Knight, J., McKenzie, C., Kranich, J., Oliveira, A. C., Rossello, F. J., Krishnamurthy, B., Nefzger, C. M., Macia, L., Thorburn, A., Baxter, A. G., Morahan, G., Wong, L. H., Polo, J. M., Moore, R. J., … Mackay, C. R. (2017). Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nature Immunology, 18(5), 552–562. https://doi.org/10.1038/ni.3713
- ^ Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK (May 2006). "Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells". Nature. 441 (7090): 235–8. Bibcode:2006Natur.441..235B. doi:10.1038/nature04753. PMID 16648838.
- ^ Jump up to: a b SÄEMANN, M. D., BÖHMIG, G. A., ÖSTERREICHER, C. H., BURTSCHER, H., PAROLINI, O., DIAKOS, C., STÖCKL, J., HÖRL, W. H., & ZLABINGER, G. J. (2000). Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. The FASEB Journal, 14(15), 2380–2382. https://doi.org/10.1096/fj.00-0359fje
- ^ Gao, Z; Yin, J; Zhang, J; Ward, R. E; Martin, R. J; Lefevre, M; Cefalu, W. T; Ye, J (2009). "Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice". Diabetes. 58 (7): 1509–17. doi:10.2337/db08-1637. PMC 2699871. PMID 19366864.
- ^ Mazidi, Mohsen; Rezaie, Peyman; Kengne, Andre Pascal; Mobarhan, Majid Ghayour; Ferns, Gordon A (2016). "Gut microbiome and metabolic syndrome". Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 10 (2): S150–7. doi:10.1016/j.dsx.2016.01.024. PMID 26916014.
- ^ Chen, Xiao; d'Souza, Roshan; Hong, Seong-Tshool (2013). "The role of gut microbiota in the gut-brain axis: Current challenges and perspectives". Protein & Cell. 4 (6): 403–14. doi:10.1007/s13238-013-3017-x. PMC 4875553. PMID 23686721.
- ^ Jump up to: a b Minemura, Masami (2015). "Gut microbiota and liver diseases". World Journal of Gastroenterology. 21 (6): 1691–702. doi:10.3748/wjg.v21.i6.1691. PMC 4323444. PMID 25684933.
- ^ Syn, Nicholas L; Teng, Michele W L; Mok, Tony S K; Soo, Ross A (2017). "De-novo and acquired resistance to immune checkpoint targeting". The Lancet Oncology. 18 (12): e731–41. doi:10.1016/s1470-2045(17)30607-1. PMID 29208439.
- ^ Jump up to: a b Moeller, Andrew H.; Li, Yingying; Ngole, Eitel Mpoudi; Ahuka-Mundeke, Steve; Lonsdorf, Elizabeth V.; Pusey, Anne E.; Peeters, Martine; Hahn, Beatrice H.; Ochman, Howard (2014-11-18). "Rapid changes in the gut microbiome during human evolution". Proceedings of the National Academy of Sciences. 111 (46): 16431–16435. Bibcode:2014PNAS..11116431M. doi:10.1073/pnas.1419136111. ISSN 0027-8424. PMC 4246287. PMID 25368157.
- ^ Engel, P.; Moran, N. (2013). "The gut microbiota of insects–diversity in structure and function". FEMS Microbiology Reviews. 37 (5): 699–735. doi:10.1111/1574-6976.12025. PMID 23692388.
- ^ Brune, A. (2014). "Symbiotic digestion of lignocellulose in termite guts". Nature Reviews Microbiology. 12 (3): 168–80. doi:10.1038/nrmicro3182. PMID 24487819. S2CID 5220210.
- ^ Jump up to: a b Dietrich, C.; Köhler, T.; Brune, A. (2014). "The cockroach origin of the termite gut microbiota: patterns in bacterial community structure reflect major evolutionary events". Applied and Environmental Microbiology. 80 (7): 2261–69. Bibcode:2014ApEnM..80.2261D. doi:10.1128/AEM.04206-13. PMC 3993134. PMID 24487532.
- ^ Jump up to: a b Mikaelyan, A.; Dietrich, C.; Köhler, T.; Poulsen, M.; Sillam-Dussès, D.; Brune, A. (2015). "Diet is the primary determinant of bacterial community structure in the guts of higher termites". Molecular Ecology. 24 (20): 5824–95. doi:10.1111/mec.13376. PMID 26348261. S2CID 206182668.
- ^ Mikaelyan, A.; Thompson, C.; Hofer, M.; Brune, A. (2016). "The deterministic assembly of complex bacterial communities in germ-free cockroach guts". Applied and Environmental Microbiology. 82 (4): 1256–63. doi:10.1128/AEM.03700-15. PMC 4751828. PMID 26655763.
Further reading[]
- Review articles
- Maranduba, Carlos Magno da Costa; De Castro, Sandra Bertelli Ribeiro; Souza, Gustavo Torres de; Rossato, Cristiano; Da Guia, Francisco Carlos; Valente, Maria Anete Santana; Rettore, João Vitor Paes; Maranduba, Claudinéia Pereira; Souza, Camila Maurmann de; Carmo, Antônio Márcio Resende do; MacEdo, Gilson Costa; Silva, Fernando de Sá (2015). "Intestinal Microbiota as Modulators of the Immune System and Neuroimmune System: Impact on the Host Health and Homeostasis". Journal of Immunology Research. 2015: 931574. doi:10.1155/2015/931574. PMC 4352473. PMID 25759850.
- De Preter, Vicky; Hamer, Henrike M; Windey, Karen; Verbeke, Kristin (2011). "The impact of pre- and/or probiotics on human colonic metabolism: Does it affect human health?". Molecular Nutrition & Food Research. 55 (1): 46–57. doi:10.1002/mnfr.201000451. PMID 21207512.
- Prakash, Satya; Rodes, Laetitia; Coussa-Charley, Michael; Tomaro-Duchesneau, Catherine; Tomaro-Duchesneau, Catherine; Coussa-Charley; Rodes (2011). "Gut microbiota: Next frontier in understanding human health and development of biotherapeutics". Biologics: Targets and Therapy. 5: 71–86. doi:10.2147/BTT.S19099. PMC 3156250. PMID 21847343.
- Wu, G. D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S. A.; Bewtra, M.; Knights, D.; Walters, W. A.; Knight, R.; Sinha, R.; Gilroy, E.; Gupta, K.; Baldassano, R.; Nessel, L.; Li, H.; Bushman, F. D.; Lewis, J.D. (2011). "Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes". Science. 334 (6052): 105–08. Bibcode:2011Sci...334..105W. doi:10.1126/science.1208344. PMC 3368382. PMID 21885731.
- Gut flora
- Bacteriology
- Digestive system
- Firmicutes
- Environmental microbiology
- Microbiomes