Iron(II) phosphate
| |

| |
| Names | |
|---|---|
| IUPAC name
Iron(II) phosphate
| |
| Other names
Ferrous phosphate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.035.456 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
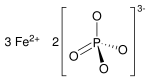
| Fe3(PO4)2 | |
| Appearance | brown powder |
| Density | 2.61 g/cm3 (octahydrate) |
| Melting point | 180 °C (356 °F; 453 K) (octahydrate) decomposes[1] |
| insoluble | |
| Structure | |
| monoclinic (octahydrate) | |
| C 2/m | |
a = 10.086 (octahydrate), b = 13.441 (octahydrate), c = 4.703 (octahydrate) α = 90°, β = 104.27°, γ = 90°
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iron(II) phosphate, also ferrous phosphate,[2] Fe3(PO4)2, is an iron salt of phosphoric acid.
Natural occurrences[]
The mineral vivianite is a naturally occurring form of hydrated iron(II) phosphate.
Production[]
It can be formed by the reaction of ferrous hydroxide with phosphoric acid to produce hydrated iron(II) phosphate.
See also[]
References[]
- ^ "iron(II) phosphate octahydrate". chemister.ru. Retrieved 2 July 2014.
- ^ "Iron(II) Phosphate". EndMemo.com. Retrieved 22 January 2016.
External links[]
![]() Media related to Iron(II) phosphate at Wikimedia Commons
Media related to Iron(II) phosphate at Wikimedia Commons
Categories:
- Phosphates
- Iron(II) compounds
- Inorganic compound stubs