Lithium–sulfur battery
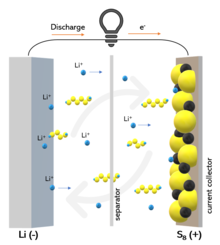 Working principle of lithium-sulfur battery and "shuttle" effect | |
| Specific energy | 450 [Wh/kg][1] |
|---|---|
| Energy density | 550 [Wh/L][1] |
| Charge/discharge efficiency | C/5 nominal |
| Cycle durability | disputed |
| Nominal cell voltage | cell voltage varies nonlinearly in the range 2.5–1.7 V during discharge; batteries often packaged for 3 V |
The lithium–sulfur battery (Li–S battery) is a type of rechargeable battery, notable for its high specific energy.[2] The low atomic weight of lithium and moderate atomic weight of sulfur means that Li–S batteries are relatively light (about the density of water). They were used on the longest and highest-altitude unmanned solar-powered aeroplane flight (at the time) by Zephyr 6 in August 2008.[3]
Lithium–sulfur batteries may succeed lithium-ion cells because of their higher energy density and reduced cost due to the use of sulfur instead of cobalt, which is commonly used in lithium-ion batteries.[4] Some Li–S batteries offer specific energies of the order of 550 Wh/kg,[1] while most lithium-ion batteries are in the range of 150–260 Wh/kg.[5] Li–S batteries with up to 1,500 charge and discharge cycles were demonstrated in 2017,[6] but cycle life tests at commercial scale and with lean electrolyte are still needed. As of early 2021, none were commercially available. The key issue of Li–S battery is the polysulfide "shuttle" effect that is responsible for the progressive leakage of active material from the cathode resulting in low life cycle of the battery.[7] Moreover, the extremely low electrical conductivity of a sulfur cathode requires an extra mass for a conducting agent in order to exploit the whole contribution of active mass to the capacity.[8] Large volume expansion of sulfur cathode from S to Li2S and the large amount of electrolyte needed are also issues to address.
History[]
The invention of Li–S batteries dates back to the 1960s, when Herbert and Ulam patented in 1962, a primary battery employing lithium or lithium alloys as anodic material, sulfur as cathodic material and an electrolyte composed of aliphatic saturated amines.[9][10] A few years later the technology was improved by the introduction of organic solvents as PC, DMSO and DMF obtaining a 2.35-2.5 V battery.[11] By the end of the 1980s a rechargeable Li–S battery was demonstrated employing ethers, in particular DOL, as the solvent for the electrolyte.[12][13] Thanks to scientific improvements in the field, the potential of Li–S batteries was highlighted. Li–S batteries have experienced in the last twenty years a renewed and growing popularity. In particular, strategies for inhibition or mitigation of the polysulfide "shuttle" effect have been deeply investigated and studied by many researchers.
Manthiram has identified the critical parameters needed for transitioning lithium sulfur batteries towards commercial use.[14][15] Specifically, lithium sulfur batteries need to achieve a sulfur loading of >5 mg cm−2, a carbon content of <5%, electrolyte-to-sulfur ratio of <5 μL mg−1, electrolyte-to-capacity ratio of <5 μL (mA h)−1, and negative-to-positive capacity ratio of <5 in pouch-type cells.[14]
As of 2017, 700 publications had appeared.[16]
Chemistry[]
Chemical processes in the Li–S cell include lithium dissolution from the anode surface (and incorporation into alkali metal polysulfide salts) during discharge, and reverse lithium plating to the anode while charging.[17]
Anode[]
At the anodic surface, dissolution of the metallic lithium occurs, with the production of electrons and lithium ions during the discharge and electrodeposition during the charge. The half-reaction is expressed as:[18]
In analogy with lithium batteries, the dissolution / electrodeposition reaction causes over time problems of unstable growth of the solid-electrolyte interface (SEI), generating active sites for the nucleation and dendritic growth of lithium. Dendritic growth is responsible for the internal short circuit in lithium batteries and leads to the death of the battery itself.[19]
Cathode[]
In Li-S batteries, energy is stored in the sulfur electrode (S8). During the discharge, the lithium ions in the electrolyte migrate to the cathode where the sulfur is reduced to lithium sulphide (Li2S). The sulfur is reoxidized to S8 during the refilling phase. The semi-reaction is therefore expressed as:
(E ° ≈ 2.15 V vs Li / Li+ )
Actually the sulfur reduction reaction to lithium sulphide is much more complex and involves the formation of lithium polysulphides (Li2Sx, 2 ≤ x ≤ 8) at decreasing chain length according to the order:[20]
The final product is actually a mixture of Li2S2 and Li2S rather than pure Li2S, due to the slow reduction kinetics at Li2S.[21] This contrasts with conventional lithium-ion cells, where the lithium ions are intercalated in the anode and cathodes. Each sulfur atom can host two lithium ions. Typically, lithium-ion batteries accommodate only 0.5–0.7 lithium ions per host atom.[22] Consequently, Li–S allows for a much higher lithium storage density. Polysulfides are reduced on the cathode surface in sequence while the cell is discharging:
- S
8 → Li
2S
8 → Li
2S
6 → Li
2S
4 → Li
2S
3
Across a porous diffusion separator, sulfur polymers form at the cathode as the cell charges:
- Li
2S → Li
2S
2 → Li
2S
3 → Li
2S
4 → Li
2S
6 → Li
2S
8 → S
8
These reactions are analogous to those in the sodium–sulfur battery.
The main challenges of Li–S batteries is the low conductivity of sulfur and its massive volume change upon discharging and finding a suitable cathode is the first step for commercialization of Li–S batteries.[23] Therefore, most researchers use a carbon/sulfur cathode and a lithium anode.[24] Sulfur is very cheap, but has practically no electroconductivity, 5×10−30 S⋅cm−1 at 25 °C.[25] A carbon coating provides the missing electroconductivity. Carbon nanofibers provide an effective electron conduction path and structural integrity, at the disadvantage of higher cost.[26]
One problem with the lithium–sulfur design is that when the sulfur in the cathode absorbs lithium, volume expansion of the LixS compositions happens, and predicted volume expansion of Li2S is nearly 80% of the volume of the original sulfur.[27] This causes large mechanical stresses on the cathode, which is a major cause of rapid degradation. This process reduces the contact between the carbon and the sulfur, and prevents the flow of lithium ions to the carbon surface.[28]
Mechanical properties of the lithiated sulfur compounds are strongly contingent on the lithium content, and with increasing lithium content, the strength of lithiated sulfur compounds improves, although this increment is not linear with lithiation.[29]
One of the primary shortfalls of most Li–S cells is unwanted reactions with the electrolytes. While S and Li
2S are relatively insoluble in most electrolytes, many intermediate polysulfides are not. Dissolving Li
2S
n into electrolytes causes irreversible loss of active sulfur.[30] Use of highly reactive lithium as a negative electrode causes dissociation of most of the commonly used other type electrolytes. Use of a protective layer in the anode surface has been studied to improve cell safety, i.e., using Teflon coating showed improvement in the electrolyte stability,[31] LIPON, Li3N also exhibited promising performance.
Polysulfide "shuttle" []
Historically, the "shuttle" effect is the main cause of degradation in a Li–S battery.[32] The lithium polysulfide Li2Sx (6≤x≤8) is highly soluble[33] in the common electrolytes used for Li–S batteries. They are formed and leaked from the cathode and they diffuse to the anode, where they are reduced to short-chain polysulfides and diffuse back to the cathode where long-chain polysulfides are formed again. This process results in the continuous leakage of active material from the cathode, lithium corrosion, low coulombic efficiency and low battery life.[34] Moreover, the "shuttle" effect is responsible for the characteristic self-discharge of Li–S batteries, because of slow dissolution of polysulfide, which occurs also in rest state.[32] The "shuttle" effect in a Li–S battery can be quantified by a factor fc (0<fc<1), evaluated by the extension of the charge voltage plateau. The factor fc is given by the expression:[35]
where ks, qup, [Stot] and Ic are respectively the kinetic constant, specific capacity contributing to the anodic plateau, the total sulfur concentration and charge current.
Electrolyte[]
Conventionally, Li–S batteries employ a liquid organic electrolyte, contained in the pores of PP separator.[32] The electrolyte plays a key role in Li–S batteries, acting both on "shuttle" effect by the polysulfide dissolution and the SEI stabilization at anode surface. It has been demonstrated that the electrolytes based on organic carbonates commonly employed in Li-ion batteries (i.e. PC, EC, DEC and mixtures of them) are not compatible with the chemistry of Li–S batteries.[36] Long-chain polysulfides undergo nucleophilic attack on electrophilic sites of carbonates, resulting in the irreversible formation of by-products as ethanol, methanol, ethylene glycol and thiocarbonates. In Li–S batteries are conventionally employed cyclic ethers (as DOL) or short-chain ethers (as DME) as well as the family of glycol ethers, including DEGDME and TEGDME.[37] One common electrolyte is 1M LiTFSI in DOL:DME 1:1 vol. with1%w/w di LiNO3 as additive for lithium surface passivation.[37]
Safety[]
Because of the high potential energy density and the nonlinear discharge and charging response of the cell, a microcontroller and other safety circuitry is sometimes used along with voltage regulators to manage cell operation and prevent rapid discharge.[38]
Research[]
| Anode | Cathode | Date | Source | Specific Capacity after cycling | Notes |
|---|---|---|---|---|---|
| Lithium metal | Polyethylene glycol coated, pitted mesoporous carbon | 17 May 2009 | University of Waterloo[39] | 1,110 mA⋅h/g after 20 cycles at a current of 168 mA⋅g−1[39] | Minimal degradation during charge cycling. To retain polysulfides in the cathode, the surface was functionalized to repel (hydrophobic) polysulfides. In a test using a glyme solvent, a traditional sulfur cathode lost 96% of its sulfur over 30 cycles, while the experimental cathode lost only 25%. |
| Lithium metal | Sulfur-coated, disordered carbon hollow carbon nanofibers | 2011 | Stanford University[40][41] | 730 mA⋅h/g after 150 cycles (at 0.5 C) | An electrolyte additive boosted the faraday efficiency from 85% to over 99%. |
| Silicon nanowire/carbon | Sulfur-coated, disordered carbon nanotubes made from carbohydrates | 2013 | CGS[42][43] | 1,300 mA⋅h/g after 400 cycles (at 1 C) | Microwave processing of materials and laser-printing of electrodes. |
| Silicon carbon | Sulfur | 2013 | Fraunhofer Institute for Material and Beam Technology IWS[44] | ? after 1,400 cycles | |
| Copolymerized sulfur | 2013 | University of Arizona[45][46] | 823 mA⋅h/g at 100 cycles | Uses "inverse vulcanization" on mostly sulfur with a small amount of 1,3-diisopropenylbenzene (DIB) additive | |
| Porous TiO 2-encapsulated sulfur nanoparticles |
2013 | Stanford University[47][48] | 721 mA⋅h/g at 1,000 cycles (0.5 C) | shell protects the sulfur-lithium intermediate from electrolyte solvent. Each cathode particle is 800 nanometers in diameter. Faraday efficiency of 98.4%. | |
| Sulfur | June 2013 | Oak Ridge National Laboratory | 1200 mA·h/g at 300 cycles at 60 °C (0.1 C)
800 mA·h/g at 300 cycles at 60 °C (1 C)[49] |
Solid lithium polysulfidophosphate electrolyte. Half the voltage of typical LIBs. Remaining issues include low electrolyte ionic conductivity and brittleness in the ceramic structure.[50][51] | |
| Lithium | Sulfur-graphene oxide nanocomposite with styrene-butadiene-carboxymethyl cellulose copolymer binder | 2013 | Lawrence Berkeley National Laboratory[52] | 700 mA·h/g at 1,500 cycles (0.05 C discharge)
400 mA·h/g at 1,500 cycles (0.5 C charge / 1 C discharge) |
Voltage between about 1.7 and 2.5 volts, depending on charge state. Lithium bis(trifluoromethanesulfonyl)imide) dissolved in a mixture of nmethyl-(n-butyl) pyrrolidinium bis(trifluoromethanesulfonyl)-imide (PYR14TFSI), 1,3-dioxolane (DOL), dimethoxyethane (DME) with 1 M lithium bis-(trifluoromethylsulfonyl)imide (LiTFSI), and lithium nitrate (LiNO 3). High porosity polypropylene separator. Specific energy is 500 W⋅h/kg (initial) and 250 W⋅h/kg at 1,500 cycles (C=1.0) |
| Lithiated graphite | Sulfur | February 2014 | Pacific Northwest National Laboratory | 400 cycles | Coating prevents polysulfides from destroying the anode.[53] |
| Lithiated graphene | Sulfur/Lithium-sulfide passivation layer | 2014 | OXIS Energy[54][55] | 240 mA·h/g (1000 cycles)
25 A·h/cell |
Passivation layer prevents sulfur loss |
| Lithiated hard-carbon | Sulfur-copolymer (poly(S-co-DVB)) | 2019 | Chungnam National University | 400 mAh/g for 500 cycles at 3C | The SEI of hard-carbon prevents polysulphides deposition at anode and enables high-rate performance.[56] |
| Lithium sulfur batteries | Carbon nanotube/Sulfur | 2014 | Tsinghua University[57] | 15.1 mA·h⋅cm−2 at a sulfur loading of 17.3 mgS⋅cm−2 | A free-standing CNT–S paper electrode with a high areal sulfur-loading was fabricated, in which short MWCNTs served as the short-range electrical conductive network and super-long CNTs acted as both the long-range conductive network and intercrossed binders. |
| Glass-coated sulfur with mildly reduced graphene oxide for structural support | 2015 | University of California, Riverside[58] | 700 mA⋅h⋅g−1 (50 cycles)[59] | Glass coating prevents lithium polysulfides from permanently migrating to an electrode | |
| Lithium | Sulfur | 2016 | LEITAT | 500 W⋅h/kg | ALISE H2020 project developing a Li–S battery for cars with new components and optimized regarding anode, cathode, electrolyte and separator |
Commercialization[]
As of 2021 few companies had been able to commercialize the technology on an industrial scale. Companies such as Sion Power have partnered with Airbus Defence and Space to test their lithium sulfur battery technology. Airbus Defense and Space successfully launched their prototype High Altitude Pseudo-Satellite (HAPS) aircraft powered by solar energy during the day and by lithium sulfur batteries at night in real life conditions during an 11-day flight. The batteries used in the test flight utilized Sion Power's Li–S cells that provide 350 W⋅h/kg.[60] Sion originally claimed to be in the process of volume manufacturing with availability by end of 2017; however more recently it can be seen that they have dropped work on their lithium sulfur battery in favor of a lithium-metal battery.[61][62]
British firm OXIS Energy developed prototype lithium sulfur batteries.[63][64] Together with Imperial College London and Cranfield University, they published equivalent-circuit-network models for its cells.[65] With Lithium Balance of Denmark they built a prototype scooter battery system primarily for the Chinese market. The prototype battery has a capacity of 1.2 kWh using 10 Ah Long Life cells, weighs 60% less than lead acid batteries with a significant increase in range.[66] They also built a 3U, 3,000 W⋅h Rack-Mounted Battery that weighs only 25 kg and is fully scalable.[67] They anticipate their Lithium-Sulfur batteries will cost about $200/kWh in mass production.[68] The firm entered bankruptcy (insolvency) status in May 2021.[69]
Sony, which also commercialized the first lithium-ion battery, planned to introduce lithium–sulfur batteries to the market in 2020, but has provided no updates since the initial announcement in 2015.[70]
Monash University’s Department of Mechanical and Aerospace Engineering in Melbourne, Australia developed an ultra-high capacity Li-S battery that has been manufactured by partners at the Fraunhofer Institute for Material and Beam Technology in Germany. It is claimed the battery can provide power to a smartphone for five days.[71]
See also[]
References[]
- ^ Jump up to: a b c "OXIS ENERGY SET TO MAKE SOLID-STATE LITHIUM-SULFUR CELL TECHNOLOGY A REALITY" (pdf). 20 April 2021. Retrieved 7 June 2021.
- ^ Zhang, Sheng S (2013). "Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions". Journal of Power Sources. 231: 153–162. doi:10.1016/j.jpowsour.2012.12.102.
- ^ Amos, J. (24 August 2008) "Solar plane makes record flight" BBC News
- ^ Manthiram, Arumugam; Fu, Yongzhu; Su, Yu-Sheng (2013). "Challenges and Prospects of Lithium–Sulfur Batteries" (PDF). Acc. Chem. Res. 46: 1125–1134. doi:10.1021/ar300179v. Archived from the original (PDF) on 2020-01-03.
- ^ Automotive Li-Ion Batteries: Current Status and Future Perspectives (Report). U.S. Department Of Energy. 2019-01-01. p. 26. Retrieved 15 March 2021.
- ^ "OXIS Energy's Lithium-Sulfur Battery Technology". Archived from the original on 28 June 2017. In 2017: "can be cycled approximately 1500 times ... In the next 2 years, we expect this to reach 2500 cycles". In 2021: "Within the next two years we aim to double the current cycle life to achieve upwards of 500 cycles"
- ^ Diao, Yan; Xie, Kai; Xiong, Shizhao; Hong, Xiaobin (August 2013). "Shuttle phenomenon – The irreversible oxidation mechanism of sulfur active material in Li–S battery". Journal of Power Sources. 235: 181–186. doi:10.1016/j.jpowsour.2013.01.132.
- ^ Eftekhari, Ali (2017). "The rise of lithium–selenium batteries". Sustainable Energy & Fuels. 1: 14–29. doi:10.1039/C6SE00094K.
- ^ US 3043896A
- ^ US 3532543A
- ^ US 3413154A
- ^ Peled, E.; Gorenshtein, A.; Segal, M.; Sternberg, Y. (May 1989). "Rechargeable lithium–sulfur battery (extended abstract)". Journal of Power Sources. 26 (3–4): 269–271. Bibcode:1989JPS....26..269P. doi:10.1016/0378-7753(89)80133-8.
- ^ Peled, E. (1989). "Lithium-Sulfur Battery: Evaluation of Dioxolane-Based Electrolytes". Journal of the Electrochemical Society. 136 (6): 1621. doi:10.1149/1.2096981.
- ^ Jump up to: a b Bhargav, Amruth; Jiarui, He (2020). "Lithium-Sulfur Batteries: Attaining the Critical Metrics". Joule. 4: 285–291. doi:10.1016/j.joule.2020.01.001.
- ^ Manthiram, Arumugam; Fu, Yongzhu; Chung, Sheng-Heng; Zu, Chenxi; Su, Yu-Sheng (2014). "Rechargeable Lithium–Sulfur Batteries". Chemical Reviews. 114: 11751–11787. doi:10.1021/cr500062v.
- ^ Kumar, Rudra; Liu, Jie; Hwang, Jang-Yeon; Sun, Yang-Kook (2018). "Recent research trends in Li–S batteries". Journal of Materials Chemistry A. 6 (25): 11582–11605. doi:10.1039/C8TA01483C. ISSN 2050-7488.
- ^ Tudron, F.B., Akridge, J.R., and Puglisi, V.J. (2004) "Lithium-Sulfur Rechargeable Batteries: Characteristics, State of Development, and Applicability to Powering Portable Electronics" (Tucson, AZ: Sion Power)
- ^ Kumar, Rudra; Liu, Jie; Hwang, Jang-Yeon (2018). "Recent research trends in Li–S batteries". Journal of Materials Chemistry A. 6 (25): 11582–11605. doi:10.1039/C8TA01483C. Retrieved 2019-07-04.
- ^ Ould Ely, Teyeb; Kamzabek, Dana; Chakraborty, Dhritiman (2018-05-29). "Lithium–Sulfur Batteries: State of the Art and Future Directions". ACS Applied Energy Materials. 1 (5): 1783–1814. doi:10.1021/acsaem.7b00153.
- ^ Lin, Zhan; Liang, Chengdu (2015). "Lithium–sulfur batteries: from liquid to solid cells". Journal of Materials Chemistry A. 3 (3): 18. doi:10.1039/C4TA04727C. OSTI 1185628. Retrieved 2019-07-04.
- ^ Song, Min-Kyu; Cairns, Elton J.; Zhang, Yuegang (2013). "Lithium/sulfur batteries with high specific energy: old challenges and new opportunities". Nanoscale. 5 (6): 2186–204. Bibcode:2013Nanos...5.2186S. doi:10.1039/c2nr33044j. PMID 23397572. Retrieved 2019-07-04.
- ^ Bullis, Kevin (May 22, 2009). "Revisiting Lithium-Sulfur Batteries". Technology Review. Retrieved August 12, 2016.
- ^ Eftekhari, A. (2017). "Cathode Materials for Lithium–Sulfur Batteries: A Practical Perspective". Journal of Materials Chemistry A. 5 (34): 17734–17776. doi:10.1039/C7TA00799J.
- ^ Choi, Y.J.; Kim, K.W. (2008). "Improvement of cycle property of sulfur electrode for lithium/sulfur battery". Journal of Alloys and Compounds. 449 (1–2): 313–316. doi:10.1016/j.jallcom.2006.02.098.
- ^ J.A. Dean, ed. (1985). Lange's Handbook of Chemistry (third ed.). New York: McGraw-Hill. pp. 3–5.
- ^ Choi, Y. J.; Chung, Y. D.; Baek, C. Y.; Kim, K. W.; Ahn, J. H. (March 4, 2008). "Effects of carbon coating on the electrochemical properties of sulfur cathode for lithium/sulfur cell". J. Power Sources. 184 (2): 548–552. Bibcode:2008JPS...184..548C. doi:10.1016/j.jpowsour.2008.02.053.
- ^ Islam, Md Mahbubul; Ostadhossein, Alireza; Borodin, Oleg; Yeates, A. Todd; Tipton, William W.; Hennig, Richard G.; Kumar, Nitin; Duin, Adri C. T. van (2015-01-21). "ReaxFF molecular dynamics simulations on lithiated sulfur cathode materials". Phys. Chem. Chem. Phys. 17 (5): 3383–3393. Bibcode:2015PCCP...17.3383I. doi:10.1039/c4cp04532g. PMID 25529209.
- ^ Brian Dodson, "New lithium/sulfur battery doubles energy density of lithium-ion", NewAtlas, 1 December 2013
- ^ Islam; et al. (2015). "ReaxFF molecular dynamics simulations on lithiated sulfur cathode materials". Phys. Chem. Chem. Phys. 17 (5): 3383–3393. Bibcode:2015PCCP...17.3383I. doi:10.1039/C4CP04532G. PMID 25529209.
- ^ Jeong, S. S.; Lim, Y.; Choi, Y. T.; Kim, K. W.; Ahn, H. J.; Cho, K. K. (2006). "Electrochemical properties of lithium sulfur cells using PEO polymer electrolytes prepared under three different mixing conditions". J. Power Sources. 174 (2): 745–750. Bibcode:2007JPS...174..745J. doi:10.1016/j.jpowsour.2007.06.108.
- ^ Islam, Md Mahbubul; Bryantsev, Vyacheslav S.; van Duin, Adri CT (2014). "ReaxFF Reactive Force Field Simulations on the Influence of Teflon on Electrolyte Decomposition during Li/SWCNT Anode Discharge in Lithium-Sulfur Batteries" (PDF). Journal of the Electrochemical Society. 161 (8): E3009–E3014. doi:10.1149/2.005408jes. Archived from the original (PDF) on 2019-02-21.
- ^ Jump up to: a b c Manthiram, Arumugam; Fu, Yongzhu; Chung, Sheng-Heng; Zu, Chenxi; Su, Yu-Sheng (2014-12-10). "Rechargeable Lithium–Sulfur Batteries". Chemical Reviews. 114 (23): 11751–11787. doi:10.1021/cr500062v. ISSN 0009-2665. PMID 25026475.
- ^ Zhang, Kintao (2018). Chemically Derived Graphene: Functionalization, Properties and Applications (illustrated ed.). Royal Society of Chemistry. p. 224. ISBN 978-1-78801-080-1. Extract of page 224
- ^ Song, Min-Kyu; Cairns, Elton J.; Zhang, Yuegang (2013). "Lithium/sulfur batteries with high specific energy: old challenges and new opportunities". Nanoscale. 5 (6): 2186–204. Bibcode:2013Nanos...5.2186S. doi:10.1039/c2nr33044j. ISSN 2040-3364. PMID 23397572.
- ^ Mikhaylik, Yuriy V.; Akridge, James R. (2004). "Polysulfide Shuttle Study in the Li/S Battery System". Journal of the Electrochemical Society. 151 (11): A1969. doi:10.1149/1.1806394.
- ^ Yim, Taeeun; Park, Min-Sik; Yu, Ji-Sang; Kim, Ki Jae; Im, Keun Yung; Kim, Jae-Hun; Jeong, Goojin; Jo, Yong Nam; Woo, Sang-Gil (September 2013). "Effect of chemical reactivity of polysulfide toward carbonate-based electrolyte on the electrochemical performance of Li–S batteries". Electrochimica Acta. 107: 454–460. doi:10.1016/j.electacta.2013.06.039.
- ^ Jump up to: a b Scheers, Johan; Fantini, Sébastien; Johansson, Patrik (June 2014). "A review of electrolytes for lithium–sulphur batteries". Journal of Power Sources. 255: 204–218. Bibcode:2014JPS...255..204S. doi:10.1016/j.jpowsour.2014.01.023.
- ^ Akridge, J.R. (October 2001) "Lithium Sulfur Rechargeable Battery Safety" Battery Power Products & Technology
- ^ Jump up to: a b Xiulei Ji, Kyu Tae Lee, and Linda F. Nazar. (17 May 2009)"A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries." Nature Materials
- ^ Guangyuan, Zheng; Yuan Yang; Judy J. Cha; Seung Sae Hong; Yi Cui (14 September 2011). "Hollow Carbon Nanofiber-Encapsulated Sulfur Cathodes for High Specific Capacity Rechargeable Lithium Batteries" (PDF). Nano Letters. 11 (10): 4462–4467. Bibcode:2011NanoL..11.4462Z. doi:10.1021/nl2027684. PMID 21916442.
- ^ Keller, Sarah Jane (October 4, 2011). "Sulfur in hollow nanofibers overcomes challenges of lithium-ion battery design". Stanford News. Stanford University. Retrieved February 18, 2012.
- ^ Rosenberg, Sarah; Hintennach (1 April 2014). "Laser-printed lithium-sulphur micro-electrodes for Li/S batteries". Russian Journal of Electrochemistry. 50 (4): 327–335. doi:10.1134/S1023193514040065.
- ^ Vandenberg, Aurelius; Hintennach (1 April 2014). "A novel design approach for lithium-sulphur batteries". Russian Journal of Electrochemistry. 50 (4): 317–326. doi:10.1134/S102319351306013X.
- ^ "Researchers increase lifespan of lithium-sulfur batteries". Gizmag.com. 4 April 2013. Retrieved 2013-12-04.
- ^ Chung, W. J.; Griebel, J. J.; Kim, E. T.; Yoon, H.; Simmonds, A. G.; Ji, H. J.; Dirlam, P. T.; Glass, R. S.; Wie, J. J.; Nguyen, N. A.; Guralnick, B. W.; Park, J.; Somogyi, Á. D.; Theato, P.; MacKay, M. E.; Sung, Y. E.; Char, K.; Pyun, J. (2013). "The use of elemental sulfur as an alternative feedstock for polymeric materials". Nature Chemistry. 5 (6): 518–524. Bibcode:2013NatCh...5..518C. doi:10.1038/nchem.1624. PMID 23695634.
- ^ Caryl Richards (2013-04-16). "Radical approach to turn sulfur into polymers".
- ^ SLAC National Accelerator Laboratory (6 Posts) (2013-01-08). "World-Record Battery Performance Achieved With Egg-Like Nanostructures". CleanTechnica. Retrieved 2013-06-11.
- ^ Wei Seh, Z.; Li, W.; Cha, J. J.; Zheng, G.; Yang, Y.; McDowell, M. T.; Hsu, P. C.; Cui, Y. (2013). "Sulphur–TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries". Nature Communications. 4: 1331. Bibcode:2013NatCo...4.1331W. doi:10.1038/ncomms2327. PMID 23299881.
- ^ Lin, Z; Liu, Z; Fu, W; Dudney, NJ; Liang, C (2013). "Lithium Polysulfidophosphates: A Family of Lithium-Conducting Sulfur-Rich Compounds for Lithium–Sulfur Batteries" (PDF). Angewandte Chemie International Edition. 52 (29): 7460–7463. doi:10.1002/anie.201300680. PMID 23737078. Archived from the original (PDF) on 2016-09-10.
- ^ Lin, Z.; Liu, Z.; Fu, W.; Dudney, N. J.; Liang, C. (2013). "Lithium Polysulfidophosphates: A Family of Lithium-Conducting Sulfur-Rich Compounds for Lithium-Sulfur Batteries" (PDF). Angewandte Chemie International Edition. 52 (29): 7460–7463. doi:10.1002/anie.201300680. PMID 23737078. Archived from the original (PDF) on 2016-09-10.
- ^ "All-solid lithium-sulfur battery stores four times the energy of lithium-ions". NewAtlas.com. 7 June 2013. Retrieved 2013-06-13.
- ^ "New lithium/sulfur battery doubles energy density of lithium-ion". NewAtlas.com. 2 December 2013. Retrieved 2013-12-04.
- ^ Lavars, Nick (February 20, 2014). "Hybrid anode quadruples the lifespan of lithium-sulfur batteries". Retrieved August 22, 2016.
- ^ "A whiff of brimstone". Economist. January 3, 2015. Retrieved August 22, 2016.
- ^ "Li–S battery company OXIS Energy reports 300 W⋅h/kg and 25 A⋅h cell, predicting 33 A⋅h by mid-2015, 500 W⋅h/kg by end of 2018". Green Car Congress. November 12, 2014. Retrieved August 22, 2016.
- ^ Nguyen, D.-T.; Hoefling, A.; Yee, M.; Nguyen, T. H. G.; Theato, P.; Lee, Y. J.; Song, S.-W. (2019). "Enabling high-rate and safe lithium ion-sulfur battery by effective combination of sulfur-copolymer cathode and hard-carbon anode". ChemSusChem. 12 (2): 480–486. doi:10.1002/cssc.201802430. PMID 30479038.
- ^ Yuan, Zhe; Peng, Hong-Jie; Huang, Jia-Qi; Liu, Xin-Yan; Wang, Dai-Wei; Cheng, Xin-Bing; Zhang, Qiang (2014-10-01). "Hierarchical Free-Standing Carbon-Nanotube Paper Electrodes with Ultrahigh Sulfur-Loading for Lithium–Sulfur Batteries" (PDF). Advanced Functional Materials. 24 (39): 6105–6112. doi:10.1002/adfm.201401501. ISSN 1616-3028. Archived from the original (PDF) on 2020-01-03.
- ^ Nealon, Sean (2015-03-03). "Glass coating for improved battery performance". R&D. Archived from the original on 2015-03-07. Retrieved August 22, 2016.
- ^ Nealon, Sean (March 2, 2015). "Glass coating improves battery performance". phys.org. Retrieved August 22, 2016.
- ^ Kopera, J (September 2014) "Sion Power's Lithium-Sulfur Batteries Power High Altitude Pseudo-Satellite Flight" Sion Power Company Press Release
- ^ "Sion Power Delivers Next Generation Battery Performance Through Patented Licerion® Technology". 2016-10-03. Retrieved 4 October 2016.
- ^ "Sion Power Announces Launch of its Groundbreaking Licerion Rechargeable Lithium Battery, Sion Power". sionpower.com.
- ^ "Anesco and OXIS to Release Lithium Sulfur Battery Storage by 2016" (press release). OXIS Energy. July 14, 2015. Retrieved August 22, 2016.
- ^ "OXIS battery powers driverless vehicle for the UK Government's Smart City Gateway programme" (press release). OXIS Energy. February 22, 2015. Archived from the original on 2016-04-29. Retrieved August 22, 2016.
- ^ Propp, K.; Marinescu, M.; Auger, D. J.; et al. (August 12, 2016). "Multi-temperature state-dependent equivalent circuit discharge model for lithium-sulfur batteries". J. Power Sources. 328: 289–299. Bibcode:2016JPS...328..289P. doi:10.1016/j.jpowsour.2016.07.090.
- ^ "Lithium Sulfur batteries will be first commercialized by 2018 in electric bikes where energy density will be improved for eventual use in electric cars". nextbigfuture.com. 2016-06-10. Retrieved 2017-02-02.
- ^ "OXIS Rack-Mounted Battery" (PDF). OXIS Energy. Retrieved May 20, 2017.
- ^ "OXIS Energy Lithium-Sulfur Battery Technology Presentation". OXIS Energy. 2016-11-03. Retrieved May 20, 2017.
- ^ "https://oxisenergy.com/". Oxis Energy. Retrieved June 7, 2021. External link in
|title=(help) - ^ "Sony battery to offer 40% longer phone life". Nikkei Asian Review. December 17, 2015. Retrieved August 22, 2016.
- ^ "'World's most efficient lithium-sulphur battery' set for launch". The Engineer. January 6, 2020. Retrieved January 9, 2020.
External links[]
- "OXIS Energy". OXIS Energy. Retrieved 2013-10-30.
- "PolyPlus Lithium Sulfur". Polyplus.com. Archived from the original on 2013-04-20. Retrieved 2013-04-06.
- "Sion Power". Sion Power. Retrieved 2013-04-06.
- "Winston Battery Limited". En.winston-battery.com. Archived from the original on 2014-03-25. Retrieved 2013-04-06.
- "EEMB Battery". EEMB Battery. Retrieved 2018-04-13.
- Rechargeable batteries
- Metal-sulfur batteries
- Lithium-ion batteries



![{\displaystyle fc={\frac {k_{\text{s}}q_{\text{up}}[S_{\text{tot}}]}{I_{c}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/92767aff703c9811fcd59e4023389b4a8bdf304f)