MXenes
In materials science, MXenes are a class of two-dimensional inorganic compounds. These materials consist of a-few-atoms-thick layers of transition metal carbides, nitrides, or . First described in 2011, MXenes combine the metallic conductivity of transition metal carbides with a hydrophilic nature because of their hydroxyl- or oxygen-terminated surfaces.[1][2]
Structure[]
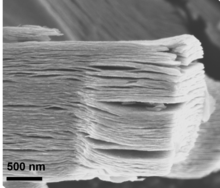
As-synthesized MXenes prepared via HF etching have an accordion-like morphology, which can be referred to as a multi-layer MXene (ML-MXene), or a few-layer MXene (FL-MXene) when there are fewer than five layers. Because the surfaces of MXenes can be terminated by functional groups, the naming convention Mn+1XnTx can be used, where T is a functional group (e.g. O, F, OH, Cl).[2]
Mono transition metal MXenes[]
MXenes adopt three structures with one metal on the M site, as inherited from the parent MAX phases: M2C, M3C2, and M4C3. They are produced by selectively etching out the A element from a MAX phase or other layered precursor (e.g., Mo2Ga2C), which has the general formula Mn+1AXn, where M is an early transition metal, A is an element from group 13 or 14 of the periodic table, X is C and/or N, and n = 1–4.[3] MAX phases have a layered hexagonal structure with P63/mmc symmetry, where M layers are nearly closed packed and X atoms fill octahedral sites.[2] Therefore, Mn+1Xn layers are interleaved with the A element, which is metallically bonded to the M element.[4][5]
Double transition metal MXenes[]
Double transition metal MXenes can take two forms, ordered double transition metal MXenes or solid solution MXenes. For ordered double transition metal MXenes, they have the general formulas: M’2M”C2 or M’2M”2C3 where M’ and M” are different transition metals. Double transition metal carbides that have been synthesized include Mo2TiC2, Mo2Ti2C3, Cr2TiC2, and Mo4VC4. In some of these MXenes (such as Mo2TiC2, Mo2Ti2C3, and Cr2TiC2), the Mo or Cr atoms are on outer edges of the MXene and these atoms control electrochemical properties of the MXenes.[6]
For solid-solution MXenes, they have the general formulas: (M’2−yM”y)C, (M’3−yM”y)C2, (M’4−yM”y)C3, or (M’5−yM”y)C4, where the metals are randomly distributed throughout the structure in solid solutions leading to continuously tailorable properties.[7]
Divacancy MXenes[]
By designing a parent 3D atomic laminate, (Mo2/3Sc1/3)2AlC, with in-plane chemical ordering, and by selectively etching the Al and Sc atoms, there is evidence for 2D Mo1.33C sheets with ordered metal divacancies.[8]
Synthesis[]

MXenes are typically synthesized by a top-down selective etching process. This synthetic route has been shown to be scalable, with no loss or change in properties as the batch size is increased.[9] Producing a MXene by etching a MAX phase occurs mainly by using strong etching solutions that contain a fluoride ion (F−) such as hydrofluoric acid (HF),[2] ammonium bifluoride (NH4HF2),[10] and a mixture of hydrochloric acid (HCl) and lithium fluoride (LiF).[11] For example, etching of Ti3AlC2 in aqueous HF at room temperature causes the A (Al) atoms to be selectively removed, and the surface of the carbide layers becomes terminated by O, OH, and/or F atoms.[12][13] MXene can also be obtained in Lewis acid molten salts, such as ZnCl2, and Cl terminal can be realized.[14] The Cl-terminated MXene is structurally stable up to 750 °C.[15] A general Lewis acid molten salt approach was proven viable to etch most of MAX phases members (such as MAX-phase precursors with A elements Si, Zn and Ga) by some other melts (CdCl2, FeCl2, CoCl2, CuCl2, AgCl, NiCl2).[16]
The MXene Ti4N3 was the first nitride MXene reported, and is prepared by a different procedure than those used for carbide MXenes. To synthesize Ti4N3, the MAX phase Ti4AlN3 is mixed with a molten eutectic fluoride salt mixture of lithium fluoride, sodium fluoride, and potassium fluoride and treated at elevated temperatures. This procedure etches out Al, yielding multilayered Ti4N3, which can further be delaminated into single and few layers by immersing the MXene in tetrabutylammonium hydroxide, followed by sonication.[17]
The following MXenes have been synthesized to date:
2-1 MXenes: Ti2C,[18] V2C,[19] Nb2C,[19] Mo2C [20] Mo2N,[21] Ti2N,[22] (Ti2−yNby)C,[7] (V2−yNby)C,[7] (Ti2−yVy)C,[7] W1.33C,[23] Nb1.33C,[24] Mo1.33C,[25] Mo1.33Y0.67C [25]
3-2 MXenes: Ti3C2 ,[1] Ti3CN,[18] Zr3C2[26] and Hf3C2[27]
4-3 MXenes: Ti4N3,[17] Nb4C3 ,[28] Ta4C3 ,[18] V4C3,[29] (Mo,V)4C3[30]
5-4 MXenes: Mo4VC4 [3]
Double transition metal MXenes:
2-1-2 MXenes: Mo2TiC2,[6] Cr2TiC2,[6] Mo2ScC2 [31]
2-2-3 MXenes: Mo2Ti2C3[6]
Covalent Surface Modification[]
2D transition-metal carbides surfaces can be chemically transformed with a variety of functional groups such as O, NH, S, Cl, Se, Br, and Te surface terminations as well as bare MXenes.[32] The strategy involves installation and removal of the surface groups by performing substitution and elimination reactions in molten inorganic salts.[33]
Intercalation and delamination[]
Since MXenes are layered solids and the bonding between the layers is weak, intercalation of the guest molecules in MXenes is possible. Guest molecules include dimethyl sulfoxide (DMSO), hydrazine, and urea.[2] For example, N2H4 (hydrazine) can be intercalated into Ti3C2(OH)2 with the molecules parallel to the MXene basal planes to form a monolayer. Intercalaction increases the MXene c lattice parameter (crystal structure parameter that is directly proportional to the distance between individual MXene layers), which weakens the bonding between MX layers.[2] Ions, including Li+, Pb2+, and Al3+, can also be intercalated into MXenes, either spontaneously or when a negative potential is applied to a MXene electrode.[34]
Delamination[]
Ti3C2 MXene produced by HF etching has accordion-like morphology with residual forces that keep MXene layers together preventing separation into individual layers. Although those forces are quite weak, ultrasound treatment results only in very low yields of single-layer flakes. For large scale delamination, DMSO is intercalated into ML-MXene powders under constant stirring to further weaken the interlayer bonding and then delaminated with ultrasound treatment. This results in large scale layer separation and formation of the colloidal solutions of the FL-MXene. These solutions can later be filtered to prepare MXene "paper" (similar to Graphene oxide paper).[35]
MXene clay[]
For the case of Ti3C2Tx and Ti2CTx, etching with concentrated hydrofluoric acid leads to open, accordion-like morphology with a compact distance between layers (this is common for other MXene compositions as well). To be dispersed in suspension, the material must be pre-intercalated with something like dimethylsulfoxide. However, when etching is conducted with hydrochloric acid and LiF as a fluoride source, morphology is more compact with a larger inter-layer spacing, presumably due to amounts of intercalated water.[11] The material has been found to be ‘clay-like’: as seen in clay materials (e.g. smectite clays and kaolinite), Ti3C2Tx demonstrates the ability to expand its interlayer distance hydration and can reversibly exchange charge-balancing Group I and Group II cations.[36] Further, when hydrated, the MXene clay becomes pliable and can be molded into desired shapes, becoming a hard solid upon drying. Unlike most clays, however, MXene clay shows high electrical conductivity upon drying and is hydrophilic, being easily dispersed into single layer two-dimensional sheets in water without surfactants. Further, due to these properties, it can be quickly rolled into free-standing, additive-free electrodes for energy storage applications.
Material processing[]
MXenes can be easily solution processed in aqueous or polar organic solvents, such as water, ethanol, dimethyl formamide, propylene carbonate, etc.,[37] enabling various types of deposition via vacuum filtration, spin coating, spray coating, dip coating, and roll casting.[38][39][40] There have been studies conducted on ink-jet printing of additive free Ti3C2Tx inks and inks composed of Ti3C2Tx and proteins.[41][42]
Lateral flake size often plays a role in the observed properties and there are several synthetic routes that produce varying degrees of flake size.[38][43] For example, when HF is used as an etchant, the intercalation and delamination step will require sonication to exfoliate material into single flakes, resulting in flakes that are several hundreds of nanometers in lateral size. This is beneficial for applications such as catalysis and select biomedical and electrochemical applications. However, if larger flakes are warranted, especially for electronic or optical applications, defect-free and large area flakes are necessary. This can be achieved by Minimally Intensive Layer Delamination (MILD) method, where the quantity of LiF to MAX phase is scaled up resulting in flakes that can be delminated in situ when washing to neutral pH.[38]
Post-synthesis processing techniques to tailor the flake size have also been investigated, such as sonication, differential centrifugation, and density gradient centrifugation procedures.[44][45] Post processing methods rely heavily on the as-produced flake size. Using sonication allows for a decrease in flake size from 4.4 μm (as-produced), to an average of 1.0 μm after 15 minutes of bath sonication (100 W, 40 kHz), down to 350 nm after 3 hours of bath sonication. By utilizing probe sonication (8 s ON, 2 s OFF pulse, 250 W), flakes were reduced to an average of 130 nm in lateral size.[44] Differential centrifugation, also known as cascading centrifugation, can be used to select flakes based on lateral size by increasing the centrifuge speed sequentially from low speeds (e.g. 1000 rpm) to high speeds (e.g., 10000 rpm) and collecting the sediment. When this was performed, "large" (800 nm), "medium" (300 nm) and "small" (110 nm) flakes can be obtained.[45] Density gradient centrifugation is also another method for selecting flakes based on lateral size, where a density gradient is employed in the centrifuge tube and flakes move through the centrifuge tube at different rates based on the flake density relative to the medium. In the case of sorting MXenes, a sucrose and water density gradient can be used from 10 to 66 w/v %.[44] Using density gradients allows for more mono-disperse distributions in flake sizes and studies show the flake distribution can be varied from 100 to 10 μm without employing sonication.[44]
Properties[]
With a high electron density at the Fermi level, MXene monolayers are predicted to be metallic.[46][47][48][49][50] In MAX phases, N(EF) is mostly M 3d orbitals, and the valence states below EF are composed of two sub-bands. One, sub-band A, made of hybridized Ti 3d-Al 3p orbitals, is near EF, and another, sub-band B, −10 to −3 eV below EF which is due to hybridized Ti 3d-C 2p and Ti 3d-Al 3s orbitals. Said differently, sub-band A is the source of Ti-Al bonds, while sub-band B is the source of Ti-C bond. Removing A layers causes the Ti 3d states to be redistributed from missing Ti-Al bonds to delocalized Ti-Ti metallic bond states near the Fermi energy in Ti2, therefore N(EF) is 2.5–4.5 times higher for MXenes than MAX phases.[1] Experimentally, the predicted higher N(EF) for MXenes has not been shown to lead to higher resistivities than the corresponding MAX phases. The energy positions of the O 2p (∼6 eV) and the F 2p (∼9 eV) bands from the Fermi level of Ti2CTx and Ti3C2Tx both depend on the adsorption sites and the bond lengths to the termination species.[51] Significant changes in the Ti-O/F coordination are observed with increasing temperature in the heat treatment.[52]
Only MXenes without surface terminations are predicted to be magnetic. Cr2C, Cr2N, and Ta3C2 are predicted to be ferromagnetic; Ti3C2 and Ti3N2 are predicted to be anti-ferromagnetic. None of these magnetic properties have yet been demonstrated experimentally.[1]
Biological properties[]
Compared to graphene oxide, which has been widely reported as an antibacterial agent, Ti2C MXene shows lack of antibacterial properties.[53] On the other hand, MXene of Ti3C2 MXene shows a higher antibacterial efficiency toward both Gram-negative E. coli and Gram-positive B. subtilis.[54] Colony forming unit and regrowth curves showed that more than 98% of both bacterial cells lost viability at 200 μg/mL Ti3C2 colloidal solution within 4 h of exposure.[54] Damage to the cell membrane was observed, which resulted in release of cytoplasmic materials from the bacterial cells and cell death.[54] The principal in vitro studies of cytotoxicity of 2D sheets of MXenes showed promise for applications in bioscience and biotechnology.[55] Presented studies of anticancer activity of the Ti3C2 MXene was determined on two normal (MRC-5 and HaCaT) and two cancerous (A549 and A375) cell lines. The cytotoxicity results indicated that the observed toxic effects were higher against cancerous cells compared to normal ones.[55] The mechanisms of potential toxicity were also elucidated. It was shown that Ti3C2 MXene may affect the occurrence of oxidative stress and, in consequence, the generation of reactive oxygen species (ROS).[55] Further studies on Ti3C2 MXene revealed potential of MXenes as a novel ceramic photothermal agent used for cancer therapy.[56] In neuronal biocompatibility studies, neurons cultured on Ti3C2 are as viable as those in control cultures, and they can adhere, grow axonal processes, and form functional networks.[57]
Water purification properties[]
One-micron-thick Ti3C2 MXene membranes demonstrated ultrafast water flux (approximately 38 L/(Bar·h·m2) and differential sieving of salts depending on both the hydration radius and charge of the ions.[58] Cations larger than the interlayer spacing of MXene do not permeate through Ti3C2 membranes.[58] As for smaller cations, the ones with a larger charge permeate an order of magnitude slower than single-charged cations.[58]
Applications[]
MXenes, as conductive layered materials with tunable surface terminations, have been shown to be promising for energy storage applications (Li-ion batteries and supercapacitors),[59] composites, photocatalysis,[60] water purification,[61] gas sensors,[62][63] transparent conducting electrodes,[39] neural electrodes,[57] as a metamaterial,[64] SERS substrate,[65] photonic diode,[66] electrochromic device,[40] and triboelectric nanogenerator (TENGs),[67] to name a few.
Lithium-ion batteries (LIBs)[]
Some MXenes have been investigated experimentally thus far in LIBs (e.g. V2CTx ,[68] Nb2CTx ,[68] Ti2CTx ,[69] and Ti3C2Tx[35]). V2CTx has demonstrated the highest reversible charge storage capacity among MXenes in multi-layer form (280 mAhg−1 at 1C rate and 125 mAhg−1 at 10C rate). Nb2CTx in multi-layer form showed a stable, reversible capacity of 170 mAhg−1 at 1C rate and 110 mAhg−1 at a 10C rate. Although Ti3C2Tx shows the lowest capacity among the four MXenes in multi-layer form, it can be easily delaminated via sonication of the multi-layer powder. By virtue of higher electrochemically active and accessible surface area, delaminated Ti3C2Tx paper demonstrates a reversible capacity of 410 mAhg−1 at 1C and 110 mAhg−1 at 36C rate. As a general trend, M2X MXenes can be expected to have greater capacity than their M3X2 or M4X3 counterparts at the same applied current, since M2X MXenes have the fewest atomic layers per sheet.
In addition to the high power capabilities of MXenes, each MXene has a different active voltage window, which could allow their use as cathodes or anodes in batteries. Moreover, the experimentally measured capacity for Ti3C2Tx paper is higher than predicted from computer simulations, indicating that further investigation is required to ascertain the charge storage mechanism on MXene surfaces.[70]
Sodium-ion batteries[]
MXenes also exhibit promising performances for sodium-based energy storage devices. Na+ should diffuse rapidly on MXene surfaces, which is favorable for fast charging/discharging.[71][72] Two layers of Na+ can be intercalated in between MXene layers.[73][74] As a typical example, multilayered Ti2CTx MXene as a negative electrode material showed a capacity of 175 mA h g−1 and good rate capability for electrochemical sodium-ion storage.[75] It is possible to tune the Na-ion insertion potentials of MXenes by changing the transition metal and surface functional groups.[71][76] V2CTx MXene has been successfully applied as a positive electrode material for sodium-ion storage.[77] Porous MXene-based paper electrodes have also been reported, which exhibited high volumetric capacities and stable cycling performance, demonstrating that MXenes are promising for sodium-based energy storage devices where size matters.[78]
Supercapacitors[]
Supercapacitor electrodes based on Ti3C2 MXene paper in aqueous solutions demonstrate excellent cyclability and the ability to store 300-400 F/cm3, which translates to three times as much energy as for activated carbon and graphene-based capacitors.[79] Ti3C2 MXene clay shows a volumetric capacitance of 900 F/cm3, a higher capacitance per unit of volume than most other materials, and does not lose any of its capacitance through more than 10,000 charge/discharge cycles.[11]
Composites[]
FL-Ti3C2 (the most studied MXene) nanosheets can mix intimately with polymers such as polyvinyl alcohol (PVA), forming alternating MXene-PVA layered structures. The electrical conductivities of the composites can be controlled from 4×10−4 to 220 S/cm (MXene weight content from 40% to 90%). The composites have tensile strength up to 400% stronger than pure MXene films and show better capacitance up to 500 F/cm3.[80] A method of alternative filtration for forming MXene-carbon nanomaterials composite films is also devised. These composites show better rate performance at high scan rates in supercapacitors.[81] The insertion of polymers or carbon nanomaterials between the MXene layers enables electrolyte ions to diffuse more easily through the MXenes, which is the key for their applications in flexible energy storage devices.
Porous MXenes[]
Porous MXenes (Ti3C2, Nb2C and V2C) have been produced via a facile chemical etching method at room temperature.[82] Porous Ti3C2 has a larger specific surface area and more open structure, and can be filtered as flexible films with, or without, the addition of carbon nanotubes (CNTs).[82] The as-fabricated p-Ti3C2/CNT films showed significantly improved lithium ion storage capabilities, with a capacity as high as 1250 mA·h·g−1 at 0.1 C, excellent cycling stability, and good rate performance.[82]
Antennas[]
Scientists at Drexel University in the US have created spray on antennas that perform as well as current antennas found in phones, routers and other gadgets by painting MXene's onto everyday objects, widening the scope of the Internet of things considerably.[83][84]
Optoelectronic devices[]
MXene SERS substrates have been manufactured by spray-coating and were used to detect several common dyes, with calculated enhancement factors reaching ~106. Titanium carbide MXene demonstrates SERS effect in aqueous colloidal solutions, suggesting the potential for biomedical or environmental applications, where MXene can selectively enhance positively charged molecules.[65] Transparent conducting electrodes have been fabricated with titanium carbide MXene showing the ability to transmit approximately 97% of visible light per nanometer thickness. The performance of MXene transparent conducting electrodes depends on the MXene composition as well as synthesis and processing parameters.[85]
Superconductivity[]
Nb2C MXenes exhibit surface-group-dependent superconductivity.[32]
References[]
- ^ Jump up to: a b c d Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. (2011). "Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2". Advanced Materials. 23 (37): 4248–4253. CiteSeerX 10.1.1.497.9340. doi:10.1002/adma.201102306. PMID 21861270.
- ^ Jump up to: a b c d e f Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. (2011). "25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials". Advanced Materials. 26 (7): 992–1005. doi:10.1002/adma.201304138. PMID 24357390.
- ^ Jump up to: a b Deysher, Grayson; Shuck, Christopher Eugene; Hantanasirisakul, Kanit; Frey, Nathan C.; Foucher, Alexandre C.; Maleski, Kathleen; Sarycheva, Asia; Shenoy, Vivek B.; Stach, Eric A.; Anasori, Babak; Gogotsi, Yury (2019). "Synthesis of Mo4VAlC4 MAX Phase and Two-Dimensional Mo4VC4 MXene with Five Atomic Layers of Transition Metals". ACS Nano. 14 (1): 204–217. doi:10.1021/acsnano.9b07708. OSTI 1774171. PMID 31804797.
- ^ Barsoum, M.W. (2000). "The Mn+1AXn Phases: a New Class of Solids; Thermodynamically Stable Nanolaminates" (PDF). Prog. Solid State Chem. 28 (1–4): 201–281. doi:10.1016/S0079-6786(00)00006-6.
- ^ Sun, Z.; Music, D.; Ahuja, R.; Li, S.; Schneider, J.M. (2004). "Bonding and classification of nanolayered ternaray carbides". Physical Review B. 70 (9): 092102. Bibcode:2004PhRvB..70i2102S. doi:10.1103/PhysRevB.70.092102. S2CID 117738466.
- ^ Jump up to: a b c d Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. (2015). "Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes)". ACS Nano. 9 (10): 9507–9516. doi:10.1021/acsnano.5b03591. PMID 26208121.
- ^ Jump up to: a b c d Han, Meikang; Maleski, Kathleen; Shuck, Christopher; Yang, Yizhou; Glazar, James; Foucher, Alexandre; Hantanasirisakul, Kanit; Sarycheva, Asia; Frey, Nathan; May, Steven; Shenoy, Vivek; Stach, Eric; Gogotsi, Yury (2020). "Tailoring Electronic and Optical Properties of MXenes through Forming Solid Solutions". Journal of the American Chemical Society. 142 (45): 19110–19118. doi:10.1021/jacs.0c07395. PMID 33108178. S2CID 225098811.
- ^ Tao, Quanzheng; Dahlqvist, Martin; Lu, Jun; Kota, Sankalp; Meshkian, Rahele; Halim, Joseph; Palisaitis, Justinas; Hultman, Lars; Barsoum, Michel W.; Persson, Per O. Å.; Rosen, Johanna (2017). "Two-dimensional Mo1.33C MXene with divacancy ordering prepared from parent 3D laminate with in-plane chemical ordering". Nature Communications. 8 (1): 14949. Bibcode:2017NatCo...814949T. doi:10.1038/ncomms14949. PMC 5413966. PMID 28440271.
- ^ Shuck, Christopher E.; Sarycheva, Asia; Anayee, Mark; Levitt, Ariana; Zhu, Yuanzhe; Uzun, Simge; Balitskiy, Vitaliy; Zahorodna, Veronika; Gogotsi, Oleksiy; Gogotsi, Yury (3 February 2020). "Scalable Synthesis of Ti3C2Tx MXene". Advanced Engineering Materials. 22 (3): 1901241. doi:10.1002/adem.201901241.
- ^ Halim, J.; Lukatskaya, M.; Cook, K.; Lu, J.; Smith, C. R.; Näslund, L.-Å.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; Barsoum, M. W. (2014). "Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films". Chemistry of Materials. 26 (7): 2374–2381. doi:10.1021/cm500641a. PMC 3982936. PMID 24741204.
- ^ Jump up to: a b c Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. (2014). "Conductive two-dimensional titanium carbide 'clay' with high volumetric capacitance". Nature. 516 (7529): 78–81. Bibcode:2014Natur.516...78G. doi:10.1038/nature13970. OSTI 1286827. PMID 25470044. S2CID 4461911.
- ^ Halim, Joseph; et al. (2016). "X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes)". Applied Surface Science. 362: 406–417. Bibcode:2016ApSS..362..406H. doi:10.1016/j.apsusc.2015.11.089.
- ^ Harris, K.J. (2015). "Direct Measurement of Surface Termination Groups and Their Connectivity in the 2D MXene V2CTx Using NMR Spectroscopy". Journal of Physical Chemistry C. 119 (24): 13713–13720. doi:10.1021/acs.jpcc.5b03038.
- ^ Li, Mian (2019). "Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes". Journal of the American Chemical Society. 141 (11): 4730–4737. arXiv:1901.05120. doi:10.1021/jacs.9b00574. PMID 30821963. S2CID 73507099.
- ^ Lu, J.; Persson, I.; Lind, H.; Li, M.; Li, Y.; Chen, K.; Zhou, J.; Du, S.; Chai, Z.; Huang, Z.; Hultman, L.; Rosen, J.; Eklund, P.; Huang, Q.; Persson, P. O. Å. (2019). "Tin+1Cn MXene with fully saturated and thermally stable Cl terminations". arXiv:1901.05212v1 [cond-mat.mtrl-sci].
- ^ Li, Youbing; Shao, Hui; Lin, Zifeng; Lu, Jun; Liu, Liyuan; Duployer, Benjamin; Persson, Per O. Å; Eklund, Per; Hultman, Lars; Li, Mian; Chen, Ke (August 2020). "A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte". Nature Materials. 19 (8): 894–899. arXiv:1909.13236. Bibcode:2020NatMa..19..894L. doi:10.1038/s41563-020-0657-0. ISSN 1476-4660. PMID 32284597. S2CID 203594112.
- ^ Jump up to: a b Urbankowski, P.; Anasori, B; Makaryan, T.; Er, D.; Kota, S.; Walsh, P.L.; Zhao, M.-Q.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. (2016-06-02). "Synthesis of two-dimensional titanium nitride Ti4N3 (MXene)". Nanoscale. 8 (22): 11385–11391. Bibcode:2016Nanos...811385U. doi:10.1039/C6NR02253G. PMID 27211286. S2CID 206040336.
- ^ Jump up to: a b c Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Gogotsi, Y.; Barsoum, M.W. (2012). "Two-Dimensional Transition metal Carbides". ACS Nano. 6 (2): 1322–1331. doi:10.1021/nn204153h. PMID 22279971. S2CID 27114444.
- ^ Jump up to: a b Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. (2013). "New Two-dimensional Niobium and Vanadium Carbides as Promising Materials for Li-ion Batteries". Journal of the American Chemical Society. 135 (43): 15966–15969. doi:10.1021/ja405735d. PMID 24144164.
- ^ Meshkian, R.; Näslund, L-Å.; Halim, J.; Lu, J.; Barsoum, M.W.; Rosen, J. (November 2015). "Synthesis of two-dimensional molybdenum carbide from the gallium based atomic laminate Mo2Ga2C". Scripta Materialia. 108: 147–150. doi:10.1016/j.scriptamat.2015.07.003.
- ^ Urbankowski, P.; Anasori, B.; Hantanasirisakul, K.; Yang, L.; Zhang, L.; Haines, B.; May, S.J.; Billinge, S.J.L.; Gogotsi, Y. (2017). "2D molybdenum and vanadium nitrides synthesized by ammoniation of 2D transition metal carbides (MXenes)". Nanoscale. 9 (45): 17722–17730. doi:10.1039/C7NR06721F. OSTI 1433989. PMID 29134998.
- ^ Soundiraraju, Bhuvaneswari; George, Benny Kattikkanal (2017). "Two-Dimensional Titanium Nitride (Ti2N) MXene: Synthesis, Characterization, and Potential Application as Surface-Enhanced Raman Scattering Substrate". ACS Nano. 11 (9): 8892–8900. doi:10.1021/acsnano.7b03129. PMID 28846394.
- ^ Meshkian, Rahele; Dahlqvist, Martin; Lu, Jun; Wickman, Björn; Halim, Joseph; Thörnberg, Jimmy; Tao, Quanzheng; Li, Shixuan; Intikhab, Saad; Snyder, Joshua; Barsoum, Michel W.; Yildizhan, Melike; Palisaitis, Justinas; Hultman, Lars; Persson, Per O. Å.; Rosen, Johanna (2018). "W-Based Atomic Laminates and Their 2D Derivative W C MXene with Vacancy Ordering". Advanced Materials. 30 (21): 1706409. doi:10.1002/adma.201706409. PMID 29633399.
- ^ Halim, J.; Palisaitis, J.; Lu, J.; Thörnberg, J.; Moon, E. J.; Precner, M.; Eklund, P.; Persson, P. O. Å.; Barsoum, M. W.; Rosen, J. (2018). "Synthesis of Two-Dimensional Nb1.33C (MXene) with Randomly Distributed Vacancies by Etching of the Quaternary Solid Solution (Nb1.33Sc0.67)AlC MAX Phase". ACS Applied Nano Materials. 1 (6): 2455–2460. doi:10.1021/acsanm.8b00332.
- ^ Jump up to: a b Persson, Ingemar; el Ghazaly, Ahmed; Tao, Quanzheng; Halim, Joseph; Kota, Sankalp; Darakchieva, Vanya; Palisaitis, Justinas; Barsoum, Michel W.; Rosen, Johanna; Persson, Per O. Å. (2018). "Tailoring Structure, Composition, and Energy Storage Properties of MXenes from Selective Etching of In-Plane, Chemically Ordered MAX Phases". Small. 14 (17): 1703676. doi:10.1002/smll.201703676. PMID 29611285.
- ^ Zhou, Jie (2016). "A Two-Dimensional Zirconium Carbide by Selective Etching of Al3C3 from Nanolaminated Zr3Al3C5". Angewandte Chemie. 128 (16): 5092–5097. doi:10.1002/ange.201510432.
- ^ Zhou, Jie; Zha, Xianhu; Zhou, Xiaobing; Chen, Fanyan; gao, guoliang; Wang, Shuwei; Shen, Cai; Chen, Tao; Zhi, Chunyi (2017). "Synthesis and Electrochemical Properties of Two-Dimensional Hafnium Carbide". ACS Nano. 11 (4): 3841–3850. doi:10.1021/acsnano.7b00030. PMID 28375599.
- ^ Ghidiu, M.; Naguib, M.; Shi, C.; Mashtalir, O.; Pan, L.M.; Zhang, B.; Yang, J.; Gogotsi, Y.; Billinge, S.J.L.; Barsoum, M.W. (2014). "Synthesis and characterization of two-dimensional Nb4C3 (MXene)". Chemical Communications. 50 (67): 9517–9520. doi:10.1039/C4CC03366C. PMID 25010704.
- ^ Tran, Minh H.; Schäfer, Timo; Shahraei, Ali; Dürrschnabel, Michael; Molina-Luna, Leopoldo; Kramm, Ulrike I.; Birkel, Christina S. (2018). "Adding a New Member to the MXene Family: Synthesis, Structure, and Electrocatalytic Activity for the Hydrogen Evolution Reaction of V4C3Tx". ACS Applied Energy Materials. 1 (8): 3908–3914. doi:10.1021/acsaem.8b00652.
- ^ Pinto, David; Anasori, Babak; Avireddy, Hemesh; Shuck, Christopher Eugene; Hantanasirisakul, Kanit; Deysher, Grayson; Morante, Joan R.; Porzio, William; Alshareef, Husam N; Gogotsi, Yury (2020). "Synthesis and Electrochemical Properties of 2D Molybdenum Vanadium Carbides – Solid Solution MXenes". Journal of Materials Chemistry A. 8 (18): 8957–8968. doi:10.1039/D0TA01798A.
- ^ Meshkian, Rahele; Tao, Quanzheng; Dahlqvist, Martin; Lu, Jun; Hultman, Lars; Rosen, Johanna (2017). "Theoretical stability and materials synthesis of a chemically ordered MAX phase, Mo2ScAlC2, and its two-dimensional derivate Mo2ScC2 MXene". Acta Materialia. 125: 476–480. Bibcode:2017AcMat.125..476M. doi:10.1016/j.actamat.2016.12.008.
- ^ Jump up to: a b Kamysbayev, Vladislav; Filatov, Alexander S.; Hu, Huicheng; Rui, Xue; Lagunas, Francisco; Wang, Di; Klie, Robert F.; Talapin, Dmitri V. (2020-07-02). "Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes". Science. 369 (6506): 979–983. Bibcode:2020Sci...369..979K. doi:10.1126/science.aba8311. ISSN 0036-8075. PMID 32616671. S2CID 220327998.
- ^ "A new strategy to synthesize 2-D inorganic materials used in capacitors, batteries, and composites". phys.org. Retrieved 2020-07-15.
- ^ Eames, C.; Islam, M.S. (2014). "Ion Intercalation into Two-Dimensional Transition-Metal Carbides: Global Screening for New High-Capacity Battery Materials" (PDF). Journal of the American Chemical Society. 136 (46): 16270–16276. doi:10.1021/ja508154e. PMID 25310601.
- ^ Jump up to: a b Mashtalir, O.; Naguib, M.; Mochalin, V.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.; Gogotsi, Y. (2013). "Intercalation and delamination of layered carbides and carbonitrides". Nature Communications. 4: 1716. Bibcode:2013NatCo...4.1716M. doi:10.1038/ncomms2664. PMID 23591883.
- ^ Ghidu, Michael (2016). "Ion-Exchange and Cation Solvation Reactions in Ti3C2 MXene". Chemistry of Materials. 28 (10): 3507–3514. doi:10.1021/acs.chemmater.6b01275.
- ^ Maleski, Kathleen; Mochalin, Vadym N.; Gogotsi, Yury (2017). "Dispersions of Two-Dimensional Titanium Carbide MXene in Organic Solvents". Chemistry of Materials. 29 (4): 1632–1640. doi:10.1021/acs.chemmater.6b04830. S2CID 99211958.
- ^ Jump up to: a b c Alhabeb, Mohamed; Maleski, Kathleen; Anasori, Babak; Lelyukh, Pavel; Clark, Leah; Sin, Saleesha; Gogotsi, Yury (2017). "Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene)". Chemistry of Materials. 29 (18): 7633–7644. doi:10.1021/acs.chemmater.7b02847. OSTI 1399240. S2CID 96438231.
- ^ Jump up to: a b Dillon, Andrew D.; Ghidiu, Michael J.; Krick, Alex L.; Griggs, Justin; May, Steven J.; Gogotsi, Yury; Barsoum, Michel W.; Fafarman, Aaron T. (2016). "Highly Conductive Optical Quality Solution-Processed Films of 2D Titanium Carbide". Advanced Functional Materials. 26 (23): 4162–4168. doi:10.1002/adfm.201600357.
- ^ Jump up to: a b Salles, Pol; Pinto, David; Hantanasirisakul, Kanit; Maleski, Kathleen; Shuck, Christopher E.; Gogotsi, Yury (2019). "Electrochromic Effect in Titanium Carbide MXene Thin Films Produced by Dip‐Coating". Advanced Functional Materials. 29 (17): 1809223. doi:10.1002/adfm.201809223.
- ^ Nicolosi, Valeria; Gogotsi, Yury; Coleman, Jonathan N.; Anasori, Babak; Nerl, Hannah C.; McEvoy, Niall; Cormac Ó Coileáin; Barwich, Sebastian; Seral‐Ascaso, Andrés (2019). "Additive-free MXene inks and direct printing of micro-supercapacitors". Nature Communications. 10 (1): 1795. Bibcode:2019NatCo..10.1795Z. doi:10.1038/s41467-019-09398-1. PMC 6470171. PMID 30996224.
- ^ Vural, Mert; Pena-Francesch, Abdon; Bars-Pomes, Joan; Jung, Huihun; Gudapati, Hemanth; Hatter, Christine B.; Allen, Benjamin D.; Anasori, Babak; Ozbolat, Ibrahim T. (2018). "Inkjet Printing of Self-Assembled 2D Titanium Carbide and Protein Electrodes for Stimuli-Responsive Electromagnetic Shielding". Advanced Functional Materials. 28 (32): 1801972. doi:10.1002/adfm.201801972.
- ^ Lipatov, Alexey; Alhabeb, Mohamed; Lukatskaya, Maria R.; Boson, Alex; Gogotsi, Yury; Sinitskii, Alexander (2016). "Effect of Synthesis on Quality, Electronic Properties and Environmental Stability of Individual Monolayer Ti3C2 MXene Flakes". Advanced Electronic Materials. 2 (12): 1600255. doi:10.1002/aelm.201600255.
- ^ Jump up to: a b c d Maleski, Kathleen; Ren, Chang E.; Zhao, Meng-Qiang; Anasori, Babak; Gogotsi, Yury (2018). "Size-Dependent Physical and Electrochemical Properties of Two-Dimensional MXene Flakes". ACS Applied Materials & Interfaces. 10 (29): 24491–24498. doi:10.1021/acsami.8b04662. PMID 29956920.
- ^ Jump up to: a b Zhang, Chuanfang John; Pinilla, Sergio; McEvoy, Niall; Cullen, Conor P.; Anasori, Babak; Long, Edmund; Park, Sang-Hoon; Seral-Ascaso, Andrés; Shmeliov, Aleksey (2017). "Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes)". Chemistry of Materials. 29 (11): 4848–4856. doi:10.1021/acs.chemmater.7b00745.
- ^ Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. (2011). "Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2". Advanced Materials. 23 (37): 4248–4253. CiteSeerX 10.1.1.497.9340. doi:10.1002/adma.201102306. PMID 21861270.
- ^ Enyashin, A.N.; Ivanovskii, A.L. (2013). "Structural and Electronic Properties and Stability of MXenes Ti2C and Ti3C2 Functionalized by Methoxy Groups". The Journal of Physical Chemistry C. 117 (26): 13637–13643. arXiv:1304.1670. doi:10.1021/jp401820b. S2CID 102267772.
- ^ Tang, Q.; Zhou, Z.; Shen, P. (2012). "Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer". Journal of the American Chemical Society. 134 (40): 16909–16916. doi:10.1021/ja308463r. PMID 22989058.
- ^ Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.-Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y. W.; Kawazoe, Y. (2013). "Novel Electronic and Magnetic Properties of Two-Dimensional Transition Metal Carbides and Nitrides". Adv. Funct. Mater. 23 (17): 2185–2192. doi:10.1002/adfm.201202502.
- ^ Xie, Y.; Kent, P.R.C. (2013). "Hybrid density functional study of structural and electronic properties of functionalized Tin+1Xn (X=C, N) monolayers". Phys. Rev. B. 87 (23): 235441. arXiv:1306.6936. Bibcode:2013PhRvB..87w5441X. doi:10.1103/PhysRevB.87.235441. S2CID 119180429.
- ^ Magnuson, M.; Halim, J.; Näslund, L.-Å. (2018). "Chemical Bonding in Carbide MXene Nanosheets". J. Elec. Spec. 224: 27–32. arXiv:1803.07502. doi:10.1016/j.elspec.2017.09.006. S2CID 4955258.CS1 maint: multiple names: authors list (link)
- ^ Magnuson, M.; Näslund, L.-Å. (2020). "Local chemical bonding and structural properties in Ti3AlC2 MAX phase and Ti3C2Tx MXene probed by Ti 1s x-ray absorption spectroscopy". Physical Review Research. 2 (3): 033516–033526. arXiv:2010.00293. Bibcode:2020PhRvR...2c3516M. doi:10.1103/PhysRevResearch.2.033516. S2CID 4955258.CS1 maint: multiple names: authors list (link)
- ^ Jastrzebska, AM.; Karwowska, E.; Wojciechowski, T.; Ziemkowska, W.; Rozmysłowska, A.; Chlubny, L.; Olszyna, A. (2018). "The Atomic Structure of Ti2C/sub> and Ti3C2 MXenes is Responsible for Their Antibacterial Activity Toward E. coli Bacteria". J. Mater. Eng. Perform. 28 (3): 1272–1277. doi:10.1007/s11665-018-3223-z.
- ^ Jump up to: a b c Rasool, K.; Helal, M.; Ali, A.; Ren, C. E.; Gogotsi, Y. (2016). "Antibacterial Activity of Ti3C2". ACS Nano. 10 (3): 3674–3684. doi:10.1021/acsnano.6b00181. PMID 26909865.
- ^ Jump up to: a b c Jastrzębska, A.; Szuplewska, A.; Wojciechowski, T.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Rozmysłowska, A.; Olszyna, A. (2017). "In vitro studies on cytotoxicity of delaminated Ti3C2 MXene". Journal of Hazardous Materials. 339: 1–8. doi:10.1016/j.jhazmat.2017.06.004. PMID 28601597.
- ^ Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. (2017). "Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion". Nano Letters. 17 (1): 384–391. Bibcode:2017NanoL..17..384L. doi:10.1021/acs.nanolett.6b04339. PMID 28026960.
- ^ Jump up to: a b Driscoll, Nicolette; Richardson, Andrew G.; Maleski, Kathleen; Anasori, Babak; Adewole, Oladayo; Lelyukh, Pavel; Escobedo, Lilia; Cullen, D. Kacy; Lucas, Timothy H. (2018). "Two-Dimensional Ti3C2 MXene for High-Resolution Neural Interfaces". ACS Nano. 12 (10): 10419–10429. doi:10.1021/acsnano.8b06014. PMC 6200593. PMID 30207690.
- ^ Jump up to: a b c Ren, C. E.; Hatzell, K. B.; Alhabeb, M.; Ling, Z.; Mahmoud, K. A.; Gogotsi, Y. (2015). "Charge- and Size-Selective Ion Sieving Through Ti3C2Tx". The Journal of Physical Chemistry Letters. 6 (20): 4026–4031. doi:10.1021/acs.jpclett.5b01895. PMID 26722772.
- ^ Ostadhossein, Alireza; Guo, Jack; Simeski, Filip; Ihme, Matthias (2019). "Functionalization of 2D materials for enhancing OER/ORR catalytic activity in Li–oxygen batteries". Communications Chemistry. 2. doi:10.1038/s42004-019-0196-2.
- ^ Mashtalir, O.; Cook, K. M.; Mochalin, V. N.; Crowe, M.; Barsoum, M. W.; Gogotsi, Y. (2014). "Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media". J. Mater. Chem. A. 2 (35): 14334–14338. doi:10.1039/C4TA02638A. S2CID 98651166.
- ^ Ren, Chang; Hatzell, Kelsey; Alhabeb, Mohamed; Ling, Zheng; Mahmoud, Khaled; Gogotsi, Yury (2015). "Charge- and Size-Selective Ion Sieving Through Ti3C2Tx MXene Membranes". The Journal of Physical Chemistry Letters. 6 (20): 4026–4031. doi:10.1021/acs.jpclett.5b01895. PMID 26722772.
- ^ Chen, J.; Chen, K.; Tong, D.; Huang, Y.; Zhang, J.; Xue, J.; Huang, Q.; Chen, T. (2014). "CO and temperature dual responsive "Smart" MXene phases". Chem. Commun. 51 (2): 314–317. doi:10.1039/C4CC07220K. PMID 25406830.
- ^ Khakbaz, Pedram; Moshayedi, Milad; Hajian, Sajjad; Soleimani, Maryam; Narakathu, Binu B.; Bazuin, Bradley J.; Pourfath, Mahdi; Atashbar, Massood Z. (2019). "Titanium Carbide MXene as NH3 Sensor: Realistic First-Principles Study". The Journal of Physical Chemistry C. 123 (49): 29794–29803. doi:10.1021/acs.jpcc.9b09823.
- ^ Chaudhuri, Krishnakali; Alhabeb, Mohamed; Wang, Zhuoxian; Shalaev, Vladimir M.; Gogotsi, Yury; Boltasseva, Alexandra (2018). "Highly Broadband Absorber Using Plasmonic Titanium Carbide (MXene)". ACS Photonics. 5 (3): 1115–1122. doi:10.1021/acsphotonics.7b01439.
- ^ Jump up to: a b Sarycheva, Asia; Makaryan, Taron; Maleski, Kathleen; Satheeshkumar, Elumalai; Melikyan, Armen; Minassian, Hayk; Yoshimura, Masahiro; Gogotsi, Yury (2017). "Two-Dimensional Titanium Carbide (MXene) as Surface-Enhanced Raman Scattering Substrate". The Journal of Physical Chemistry C. 121 (36): 19983–19988. doi:10.1021/acs.jpcc.7b08180. OSTI 1399222.
- ^ Dong, Yongchang; Chertopalov, Sergii; Maleski, Kathleen; Anasori, Babak; Hu, Longyu; Bhattacharya, Sriparna; Rao, Apparao M.; Gogotsi, Yury; Mochalin, Vadym N. (2018). "Saturable Absorption in 2D Ti3C2 MXene Thin Films for Passive Photonic Diodes". Advanced Materials. 30 (10): 1705714. doi:10.1002/adma.201705714. PMID 29333627.
- ^ Dong, Yongchang; Mallineni, Sai Sunil Kumar; Maleski, Kathleen; Behlow, Herbert; Mochalin, Vadym N.; Rao, Apparao M.; Gogotsi, Yury; Podila, Ramakrishna (2018). "Metallic MXenes: A new family of materials for flexible triboelectric nanogenerators". Nano Energy. 44: 103–110. doi:10.1016/j.nanoen.2017.11.044.
- ^ Jump up to: a b Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. (2013). "New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries". Journal of the American Chemical Society. 135 (43): 15966–15969. doi:10.1021/ja405735d. PMID 24144164.
- ^ Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.-L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. (2012). "MXene: a promising transition metal carbide anode for lithium-ion batteries" (PDF). Electrochemistry Communications. 16 (1): 61–64. doi:10.1016/j.elecom.2012.01.002.
- ^ Xie, Yu; Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y.; Yu, X.; Nam, K.-W.; Yang, X.-Q.; Kolesnikov, A.I.; Kent, P.R.C. (2014). "Role of Surface Structure on Li-Ion Energy Storage Capacity of Two-Dimensional Transition-Metal Carbides". Journal of the American Chemical Society. 136 (17): 6385–6394. doi:10.1021/ja501520b. PMID 24678996.
- ^ Jump up to: a b Yang, E.; Ji, H.; Kim, J.; Jung, Y. (2015-02-04). "Exploring the possibilities of two-dimensional transition metal carbides as anode materials for sodium batteries". Phys. Chem. Chem. Phys. 17 (7): 5000–5005. Bibcode:2015PCCP...17.5000Y. doi:10.1039/C4CP05140H. PMID 25591787. S2CID 46155966.
- ^ Er, D.; Li, J.; Naguib, M.; Gogotsi, Y.; Shenoy, V.B. (2014). "Ti3C2 MXene as a High Capacity Electrode Material for Metal (Li, Na, K, Ca) Ion Batteries". ACS Applied Materials & Interfaces. 6 (14): 11173–11179. doi:10.1021/am501144q. PMID 24979179.
- ^ Xie, Y.; Dall'Agnese, Y.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W.; Zhuang, H.L.; Kent, P.R.C. (2014). "Prediction and Characterization of MXene Nanosheet Anodes for Non-Lithium-Ion Batteries". ACS Nano. 8 (9): 9606–9615. doi:10.1021/nn503921j. PMID 25157692.
- ^ Wang, X.; Shen, X.; Gao, Y.; Wang, Z.; Yu, R.; Chen, L. (2015). "Atomic-Scale Recognition of Surface Structure and Intercalation Mechanism of Ti3C2X". Journal of the American Chemical Society. 137 (7): 2715–2721. doi:10.1021/ja512820k. PMID 25688582.
- ^ Wang, X.; Shen, X.; Gao, Y.; Wang, Z.; Yu, R.; Chen, L. (2015-04-02). "Pseudocapacitance of MXene nanosheets for high-power sodium-ion hybrid capacitors". Nat. Commun. 6: 6544. Bibcode:2015NatCo...6.6544W. doi:10.1038/ncomms7544. PMC 4396360. PMID 25832913.
- ^ Eames, C.; Islam, M.S. (2014). "Ion Intercalation into Two-Dimensional Transition-Metal Carbides: Global Screening for New High-Capacity Battery Materials". Journal of the American Chemical Society. 136 (46): 16270–16276. doi:10.1021/ja508154e. PMID 25310601.
- ^ Dall'Agnese, Y.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. (2015). "Two-Dimensional Vanadium Carbide (MXene) as Positive Electrode for Sodium-Ion Capacitors" (PDF). The Journal of Physical Chemistry Letters. 6 (12): 2306–2309. doi:10.1021/acs.jpclett.5b00868. PMID 26266609.
- ^ Xie, X.; Zhao, M.-Q.; Anasori, B.; Maleski, K.; Ren, E.; Li, C.E.; Li, J.; Byles, B.W.; Pomerantseva, E.; Wang, G.; Gogotsi, Y. (August 2016). "Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices". Nano Energy. 26: 513–523. doi:10.1016/j.nanoen.2016.06.005.
- ^ Lukatskaya, M. R.; Mashtalir, O.; Ren, C. E.; Dall'Agnese, Y.; Rozier, P.; Taberna, P. L.; Naguib, M.; Simon, P.; Barsoum, M. W.; Gogotsi, Y. (2013). "Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide" (PDF). Science. 341 (6153): 1502–1505. Bibcode:2013Sci...341.1502L. doi:10.1126/science.1241488. PMID 24072919. S2CID 206550306.
- ^ Ling, Z.; Ren, C. E.; Zhao, M.-Q.; Yang, J.; Giammarco, J. M.; Qiu, J.; Barsoum, M. W.; Gogotsi, Y. (2014). "Flexible and conductive MXene films and nanocomposites with high capacitance". Proceedings of the National Academy of Sciences. 111 (47): 16676–16681. Bibcode:2014PNAS..11116676L. doi:10.1073/pnas.1414215111. PMC 4250111. PMID 25389310.
- ^ Zhao, M.-Q.; Ren, C. E.; Ling, Z.; Lukatskaya, M. R.; Zhang, C.; Van Aken, K. L.; Barsoum, M. W.; Gogotsi, Y. (2015). "Flexible MXene/Carbon Nanotube Composite Paper with High Volumetric Capacitance". Advanced Materials. 27 (2): 339–345. doi:10.1002/adma.201404140. OSTI 1265885. PMID 25405330.
- ^ Jump up to: a b c Ren, C. E.; Zhao, M.-Q.; Makaryan, T.; Halim, J.; Boota, M.; Kota, S.; Anasori, B.; Barsoum, M.W.; Gogotsi, Y. (2016). "Porous Two-Dimensional Transition Metal Carbide (MXene) Flakes for High-Performance Li-Ion Storage". ChemElectroChem. 3 (5): 689–693. doi:10.1002/celc.201600059. OSTI 1261374.
- ^ Sarycheva, A.; Polemi, A.; Liu, Y.; Dandekar, K.; Anasori, B.; Gogotsi, Y. (2018). "2D titanium carbide (MXene) for wireless communication". Science Advances. 4 (9): aau0920. Bibcode:2018SciA....4..920S. doi:10.1126/sciadv.aau0920. PMC 6155117. PMID 30255151.
- ^ Han, Meikang; Liu, Yuqiao; Rakhmanov, Roman; Israel, Christopher; Tajin, Md Abu Saleh; Friedman, Gary; Volman, Vladimir; Hoorfar, Ahmad; Dandekar, Kapil R.; Gogotsi, Yury (January 2021). "Solution‐Processed Ti 3 C 2 T x MXene Antennas for Radio‐Frequency Communication". Advanced Materials. 33 (1): 2003225. doi:10.1002/adma.202003225. PMID 33251683. S2CID 227235744.
- ^ Hantanasirisakul, Kanit; Alhabeb, Mohamed; Lipatov, Alexey; Maleski, Kathleen; Anasori, Babak; Salles, Pol; Ieosakulrat, Chanoknan; Pakawatpanurut, Pasit; Sinitskii, Alexander (2019). "Effects of Synthesis and Processing on Optoelectronic Properties of Titanium Carbonitride MXene". Chemistry of Materials. 31 (8): 2941–2951. doi:10.1021/acs.chemmater.9b00401. OSTI 1774175.
- Materials science
- Electrochemistry
- Physical chemistry
- Inorganic carbon compounds