Oligodendrocyte progenitor cell
| Oligodendrocyte progenitor cell | |
|---|---|
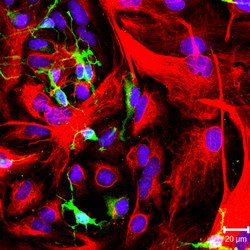 NG2-positive oligodendrocyte progenitor cells (green color) and GFAP-positive astrocytes (red color) in culture. | |
| Details | |
| Location | Central nervous system |
| Identifiers | |
| Latin | Cellula oligodendrocytoprogenetrix |
| Acronym(s) | OPC |
| TH | H2.00.06.2.01007 |
| Anatomical terms of microanatomy | |
Oligodendrocyte progenitor cells (OPCs), also known as oligodendrocyte precursor cells, NG2-glia or polydendrocytes, are a subtype of glial cells in the central nervous system.[1] They are process-bearing glial cells (neuroglia) in the mammalian central nervous system (CNS) that are identified by the expression of the NG2 chondroitin sulfate proteoglycan (CSPG4) [2] and the alpha receptor for platelet-derived growth factor (PDGFRA).[3] They are precursors to oligodendrocytes and may also be able to differentiate into neurons and astrocytes.[citation needed]
Differentiated oligodendrocytes support axons and provide electrical insulation in the form of a myelin sheath, enabling faster action potential propagation and high fidelity transmission without a need for an increase in axonal diameter.[4] A subpopulation of polydendrocytes in the gray matter of the embryonic CNS also generates .
The loss or lack of OPCs, and consequent lack of differentiated oligodendrocytes, is associated with a loss of myelination and subsequent impairment of neurological functions.[5] In addition, polydendrocytes express receptors for various neurotransmitters and undergo membrane depolarization when they receive synaptic inputs from neurons.
Structure[]
Oligodendrocyte progenitor cells are a subtype of glial cells in the central nervous system, characterized by expression of the proteoglycans PDGFRA, and CSPG4.[1] OPCs are smaller than neurons, of comparable size to other glia, and can either have a bipolar or complex multipolar morphology with processes reaching up to ~50 μm.[6]
OPCs encompass approximately 3-4% of cells in the grey matter and 8-9% in white matter, making them the fourth largest group of glia after astrocytes, microglia and oligodendrocytes.[7]
OPCs are particularly prevalent in the hippocampus and in all layers of the neocortex.[8][9] In white matter, OPCs are found along unmyelinated axons[10] as well as along myelinated axons, engulfing nodes of Ranvier.[11][12] Recently, OPCs have been shown to reside in close contact with NG2-expressing pericytes in cerebral white matter, as well.[13]
OPCs have a remarkable homogenic distribution throughout the brain. This is achieved through active self-repulsion, causing the cells to be generally equally spaced from one another.[6][14] OPCs constantly survey their surroundings through actively extending and retracting processes that have been termed growth cone like processes.[15] Death or differentiation of an OPC is rapidly followed by migration or local proliferation of a neighbouring cell.
OPCs receive synaptic contacts onto their processes from both glutamatergic[16] and GABAergic neurons.[1][17] OPCs receive preferred somatic contacts from fast-spiking GABAergic neurons, while non-fast spiking interneurons have a preference for contacting the processes.[18] These inhibitory connections (in mice) occur mainly during a specific period in development, from postnatal day 8 till postnatal day 13.
Development[]
OPCs originate in the neuroepithelium of the spine and migrate to other areas of the brain.[19] Several waves of OPC production and migration lead to the generation of oligodendrocytes.[20] OPCs are highly proliferative, migratory and bipolar.[21] The first wave of OPC production originates in the ganglionic eminence.
As development progresses a second and third wave of OPCs originate from the lateral and caudal ganglionic eminences and generate the majority of adult oligodendrocytes.[22] OPCs then migrate across most of the developing brain and spinal cord and eventually myelinate the entire central nervous system (CNS).[23] They differentiate into the less mobile, pro-oligodendrocytes that further differentiate into oligodendrocytes, a process characterized by the emergence of the expression of myelin basic protein (MBP), proteolipid protein (PLP), or myelin-associated glycoprotein (MAG).[21] Following terminal differentiation in vivo, mature oligodendrocytes wrap around and myelinate axons. In vitro, oligodendrocytes create an extensive network of myelin-like sheets. The process of differentiation can be observed both through morphological changes and cell surface markers specific to the discrete stage of differentiation, though the signals for differentiation are unknown.[24] The various waves of OPCs could myelinate distinct regions of the brain, which suggests that distinct functional subpopulations of OPCs perform different functions.[25]
OPCs are found in both white and gray matter. However, the number of OPCs is higher in white matter than in gray matter because of a higher rate of proliferation in the former. White matter OPCs proliferate and contribute to adult oligodendrogenesis, while gray matter OPCs are slowly proliferative or quiescent and mostly remain in an immature state.[26] White and gray matter OPCs have different resting membrane potentials and ion channel expression. Gray matter lacks voltage-gated sodium channels while white matter does not and produces action potentials. Cells that produce action potentials can receive signals from other neurons.[27] These differences in OPC function depend on their locations.[28]
Through maturation, OPCs are produced in the sub-ventricular zone (SVZ). The stem cells in the SVZ generate C cells which produce OPCs that go into the olfactory bulb.[29] The number of oligodendrocytes that are later formed depends on the part of the SVZ they came from. More oligodendrocytes are produced from the dorsal part of the SVZ than the ventrolateral part, and more are produced from the posterior than the rostral part.[30][31] This is due to differing environmental factors in these locations. The Wnt in the dorsal part favors OPC specification and the Bmp in the ventral part inhibits it.[32] These molecules help cause the expression of certain transcription factors.
Expression of Olig2 generates motor neurons and OPCs, dependent on the Shh and regulated by the Notch signaling pathway. This regulation limits the number of motor neurons and allows more oligodendrocytes to be produced.[33][34] is one of the most important transcription factors involved in oligodendrocyte production. Olig2 inactivation during development reduces OPC production.[35]
Differentiation of OPCs into oligodendrocytes involves massive reorganization of cytoskeleton proteins ultimately resulting in increased cell branching and lamella extension, allowing oligodendrocytes to myelinate multiple axons.[21] Laminin, a component of the extracellular matrix, plays an important role regulating oligodendrocyte production. Mice lacking laminin alpha2-subunit produced fewer OPCs in the SVZ.[36] MicroRNA (miRNA) plays a role in the regulation of oligodendrocyte differentiation and myelin maintenance. Deletion of Dicer1 in miRNA disrupts normal brain myelination. However, miR-7a, and miRNA in OPCs, promotes OPC production during brain development.[37]
Multiple pathways cause oligodendrocyte branching, but their specific contributions have yet to be resolved and the process by which oligodendrocytes extend and wrap around multiple axons remains poorly understood.[21]
Origin[]
In the embryonic spinal cord, a major source of polydendrocytes is the ventral ventricular zone of the pMN domain marked by the expression of the transcription factors Olig1 and Olig2 and the p3 domain that expresses Nkx2.2, which are induced by the morphogen Shh (sonic hedgehog). Some polydendrocytes also arise from the dorsal ventricular zone. In the forebrain, three regionally distinct sources have been shown to generate polydendrocytes sequentially: an early ventral source from the medial ganglionic eminence marked by Nkx2.1, followed by progenitor cells in the lateral ganglionic eminence marked by Gsh2, and finally the dorsal neocortical germinal zone marked by Emx1.[38] After the committed progenitor cells exit the germinal zones, they begin to express NG2 and Pdgfra and expand by local proliferation and migration and eventually occupy the entire CNS parenchyma. Polydendrocytes continue to exist in the adult CNS and retain their proliferative ability throughout life.
Fate[]
The fate of polydendrocytes has been highly debated.[39] Using Cre-Lox recombination-mediated genetic fate mapping, several labs have reported the fate of polydendrocytes using different Cre driver and reporter mouse lines;[40][41][42][43] reviewed in reference.[44] The consensus of these studies is that polydendrocytes generate predominantly oligodendrocytes in both gray and white matter. The rate at which they generate oligodendrocytes declines with age and is greater in white matter than in gray matter. These studies revealed that up to 30% of the oligodendrocytes that exist in the adult corpus callosum are generated de novo from polydendrocytes over a period of 2 months. It is not known whether all polydendrocytes eventually generate oligodendrocytes while self-renewing its population or whether some remain as polydendrocytes throughout the life of the animal and never differentiate into oligodendrocytes.
Using NG2cre mice, it was shown that polydendrocytes in the prenatal and perinatal gray matter of the ventral forebrain and spinal cord generate protoplasmic astrocytes in addition to oligodendrocytes. However, contrary to the prediction from optic nerve cultures, polydendrocytes in white matter do not generate astrocytes. When the oligodendrocyte transcription factor Olig2 is deleted specifically in polydendrocytes, there is a region- and age-dependent switch in the fate of polydendrocytes from oligodendrocytes to astrocytes.[45]
Although controversy still continues about the neuronal fate of polydendrocytes, the consensus from a number of recent genetic fate mapping studies described above seems to be that polydendrocytes do not generate a significant number of neurons under normal conditions, and that they are distinct from neural stem cells that reside in the subventricular zone.[46]
Function[]
OPCs were long held to function purely as progenitors to oligodendrocytes, hence the name. Later, additional functions were suggested.
The primary function is to serve as precursor for oligodendrocytes as well as some protoplasmic astrocytes in grey matter.[47] Postnatally, OPCs remain lineage-restricted and generally only differentiate into oligodendrocytes.
Whereas some studies suggested that OPCs can generate cortical neurons, other studies rejected these findings.[48] The question is unresolved, as studies continue to find that certain populations of OPCs can form neurons.[49]
OPCs synthesize the neuromodulatory factors prostaglandin D2 synthase (PTGDS) and neuronal pentraxin 2 (Nptx2).[50] This is mediated by the protein NG2, whose intracellular domain can be cleaved by the γ-secretase[51][52] and translocated to the nucleus.
The two N-terminal LNS (laminin/neurexin/sex hormone-binding globulin-domain) domains of the NG2 ectodomain can modulate signalling via AMPA and NMDA receptors of neuronal synapses within the cortex, including neuronal LTP. The NG2 ectodomain is released into the ECM from the full-length NG2 protein by constitutive and activity-dependent activity of the ADAM10 protease (α-secretase activity), showing that NG2 can modulate the neuronal glutamatergic system.[51][52]
Recent work has also illustrated that OPCs can act as antigen presenting cells.[53] They have been shown to express functional MHC II and initiate a learnt CD4+ immunological response.
Remyelination[]
Spontaneous myelin repair was first observed in cat models.[54] It was later discovered to occur in the human CNS as well, specifically in cases of multiple sclerosis (MS).[55] Spontaneous myelin repair does not result in morphologically normal oligodendrocytes and is associated with thinner myelin compared to axonal diameter than normal myelin.[56] Despite morphological abnormalities, however, remyelination does restore normal conduction.[57] In addition, spontaneous remyelination does not appear to be rare, at least in the case of MS. Studies of MS lesions reported the average extent of remyelination as high as 47%.[58] Comparative studies of cortical lesions reported a greater proportion of remyelination in the cortex as opposed to white matter lesions.[55]
Polydendrocytes retain the ability to proliferate in adulthood and comprise 70-90% of the proliferating cell population in the mature CNS.[59][60] Under conditions in the developing and mature CNS where a reduction in the normal number of oligodendrocytes or myelin occurs, polydendrocytes react promptly by undergoing increased proliferation. In acute or chronic demyelinated lesions created in the rodent CNS by chemical agents such as lysolecithin or cuprizone, polydendrocytes proliferate in response to demyelination, and the proliferated cells differentiate into remyelinating oligodendrocytes.[61][62] Similarly, polydendrocyte proliferation occurs in other types of injury that are accompanied by loss of myelin, such as spinal cord injury.[63]
If polydendrocytes were capable of giving rise to myelinating oligodendrocytes, one would expect complete remyelination of pathologically demyelinated lesions such as those seen in multiple sclerosis (MS). However, complete myelin regeneration is usually not observed clinically or in chronic experimental models. Possible explanations for remyelination failure include depletion of polydendrocytes over time, failure to recruit polydendrocytes to the demyelinated lesion, and failure of recruited polydendrocytes to differentiate into mature oligodendrocytes.[63]
Numerous factors have been shown to regulate polydendrocyte proliferation, migration, and differentiation [63] (reviewed in [64][65][66]). In fresh MS lesions, clusters of HNK-1+ oligodendrocytes have been observed,[67] which suggests that under favorable conditions polydendrocytes expand around demyelinated lesions and generate new oligodendrocytes. In chronic MS lesions where remyelination is incomplete, there is evidence that there are oligodendrocytes with processes extending toward demyelinated axons, but they do not seem to be able to generate new myelin.[68] The mechanisms that regulate differentiation of polydendrocytes into myelinating oligodendrocytes are an actively investigated area of research.
Another unanswered question is whether the polydendrocyte pool eventually becomes depleted after they are used to generate remyelinating cells. Clonal analysis of isolated polydendrocytes in the normal mouse forebrain suggests that in the adult, most clones originating from single polydendrocytes consist of either a heterogeneous population containing both oligodendrocytes and polydendrocytes or consist exclusively of polydendrocytes, suggesting that polydendrocytes in the adult CNS are able to self-renew and are not depleted under normal conditions.[69] However, it is not known whether this dynamic is altered in response to demyelinating lesions.
Neuron-polydendrocyte interactions[]
There is substantial evidence that indicates a functional interaction between polydendrocytes and neurons.
Node of Ranvier[]
Polydendrocytes extend their processes to the nodes of Ranvier[70] and together with astrocyte processes make up the nodal glial complex. Since the nodes of Ranvier contain a high density of voltage-dependent sodium channels and allow regenerative action potentials to be generated, it is speculated that this location allows polydendrocytes to sense and possibly respond to neuronal activity
Neuron-polydendrocyte synapse[]
Studies have shown that neurons form synapses with polydendrocytes in both gray matter[71] and white matter.[70][72] Polydendrocytes express the AMPA type glutamate receptors and GABAA receptors and undergo small membrane depolarizations when stimulated by glutamate or GABA that is vesicularly released from presynaptic terminals. Electron microscopy revealed polydendrocyte membranes apposed to neuronal presynaptic terminals filled with synaptic vesicles. Polydendrocytes lose their ability to respond to synaptic inputs from neurons as they differentiate into mature oligodendrocytes.[73][74]
Polydendrocytes can undergo cell division while maintaining synaptic inputs from neurons.[75] These observations suggest that cells that receive neuronal synaptic inputs and those that differentiate into oligodendrocytes are not mutually exclusive cell populations but that the same population of polydendrocytes can receive synaptic inputs and generate myelinating oligodendrocytes. The functional significance of the neuron-polydendrocyte synapses remains to be elucidated.
Cell types[]
Mature oligodendrocytes are unlikely to contribute to spontaneous remyelination even if they survive the original demyelinating injury.[76] New oligodendrocytes have been observed in areas of myelin damage, although the source of these new cells is unresolved. One possibility is that mature oligodendrocytes from uninjured areas migrate to the injury site and engage in myelination. This is unlikely because the transplantation of mature human oligodendrocytes achieve minimal myelin formation in the demyelinated rodent CNS. Another possibility is that mature oligodendrocytes de-differentiate into OPCs that then proliferate and remyelinate, Little experimental evidence supports this view.[citation needed]
Source of new oligodendrocytes[]
Some evidence suggests that the source of these new oligodendrocytes is proliferative adult oligodendrocyte precursor cells. Such cells have been demonstrated to exist in the adult rodent[77] and human CNS.[78] These oligodendrocyte precursor cells appear to play a major role in remyelination and are, unlike mature oligodendrocytes, able to cause extensive remyelination after transplantation into areas of myelin damage.[79] The role of these cells absent local demyelination, however, is under investigation. The fact that oligodendrocyte progenitors exhibit electrophysiological properties related to the expression of a range of glutamate receptors allowing communication with the neuron-axon unit suggests that OPCs are likely to have additional functions.[80]
The observation of OPCs in MS lesions that have not remyelinated suggested the hypothesis that the differentiation of these progenitors has been inhibited. One proposed mechanism involves the accumulation of myelin debris at the axon, suggesting that the inflammatory environment may be conducive to remyelination, as does the release of growth factors by inflammatory cells and activated microglia.[81] Alternatively, the accumulation of glycosaminoglycan hyaluronan at the lesion site may inhibit OPC differentiation. The release of OPC-specific antibodies by chronically demyelinated axons have been implicated as a remyelination inhibitor.[82] Other proposed mechanisms posit that OPC migration is inhibited by either molecules expressed by chronically demyelinated axons or the accumulation of unreactive astrocytes in MS lesions.[82]
Transplantation[]
OPC transplants contribute to remyelination, but it is difficult to maintain such cells in adequate concentrations at high purity. Finding a source for these cells remains impractical as of 2016. Should adult cells be used for transplantation, a brain biopsy would be required for each patient, adding to the risk of immune rejection. Embryonically derived stem cells have been demonstrated to carry out remyelination under laboratory conditions, but some religious groups are opposed to their use. Adult central nervous system stem cells have also been shown to generate myelinating oligodendrocytes, but are not readily accessible.[83]
Even if a viable source of OPCs were found, identifying and monitoring the outcome of remyelination remains difficult, though multimodal measures of conduction velocity and emerging magnetic resonance imaging techniques offer improved sensitivity versus other imaging methods.[84] In addition, the interaction between transplanted cells and immune cells and the effect of inflammatory immune cells on remyelination have yet to be fully characterized. If the failure of endogenous remyelination is due to an unfavorable differentiation environment, then this will have to be addressed prior to transplantation.
History[]
It had been known since the early 1900s that astrocytes, oligodendrocytes, and microglia make up the major glial cell populations in the mammalian CNS. The presence of another glial cell population had escaped recognition because of the lack of a suitable marker to identify them in tissue sections. The notion that there exists a population of glial progenitor cells in the developing and mature CNS began to be entertained in the late 1980s by several independent groups. In one series of studies on the development and origin of oligodendrocytes in the rodent CNS, a population of immature cells that appeared to be precursors to oligodendrocytes was identified by the expression of the GD3 ganglioside.[85][86]
In a separate series of studies, cells from perinatal rat optic nerves that expressed the A2B5 ganglioside were shown to differentiate into oligodendrocytes in culture.[87] Subsequently, A2B5+ cells from other CNS regions and from adult CNS were also shown to generate oligodendrocytes. Based on the observation that these cells require PDGF for their proliferation and expansion, the expression of the alpha receptor for platelet-derived growth factor (Pdgfra) was used to search for the in vivo correlates of the A2B5+ cells, which led to the discovery of a unique population of Pdgfra+ cells in the CNS whose appearance and distribution were consistent with those of developing oligodendrocytes.[88]
Independently, Stallcup and colleagues generated an antiserum that recognized a group of rat brain tumor cell lines, which exhibited properties that were intermediate between those of typical neurons and glial cells. Biochemical studies showed that the antiserum recognized a chondroitin sulfate proteoglycan with a core glycoprotein of 300 kDa,[89] and the antigen was named NG2 (nerve/glial antigen 2).[90][91] NG2 was found to be expressed on A2B5+ oligodendrocyte precursor cells isolated from the perinatal rat CNS tissues and on process-bearing cells in the CNS in vivo.[89][92] Comparison of NG2 and Pdgfra expression revealed that NG2 and Pdgfra are expressed on the same population of cells in the CNS.[93] These cells represent 2-9% of all the cells and remain proliferative in the mature CNS.[59]
See also[]
References[]
- ^ Jump up to: a b c Nishiyama A, Komitova M, Suzuki R, Zhu X (January 2009). "Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity". Nature Reviews. Neuroscience. 10 (1): 9–22. doi:10.1038/nrn2495. PMID 19096367. S2CID 15264205.
- ^ Ensembl genome browser 68: Homo sapiens - Result in Detail - Ensembl Lucene search
- ^ Ensembl genome browser 68: Homo sapiens - Result in Detail - Ensembl Lucene search
- ^ Swiss VA, Nguyen T, Dugas J, Ibrahim A, Barres B, Androulakis IP, Casaccia P (April 2011). Feng Y (ed.). "Identification of a gene regulatory network necessary for the initiation of oligodendrocyte differentiation". PLOS ONE. 6 (4): e18088. Bibcode:2011PLoSO...618088S. doi:10.1371/journal.pone.0018088. PMC 3072388. PMID 21490970.
- ^ Buller B, Chopp M, Ueno Y, Zhang L, Zhang RL, Morris D, Zhang Y, Zhang ZG (December 2012). "Regulation of serum response factor by miRNA-200 and miRNA-9 modulates oligodendrocyte progenitor cell differentiation". Glia. 60 (12): 1906–14. doi:10.1002/glia.22406. PMC 3474880. PMID 22907787.
- ^ Jump up to: a b Hughes EG, Kang SH, Fukaya M, Bergles DE (June 2013). "Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain". Nature Neuroscience. 16 (6): 668–76. doi:10.1038/nn.3390. PMC 3807738. PMID 23624515.
- ^ Dawson MR, Polito A, Levine JM, Reynolds R (October 2003). "NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS". Molecular and Cellular Neurosciences. 24 (2): 476–88. doi:10.1016/S1044-7431(03)00210-0. PMID 14572468. S2CID 21910392.
- ^ Ong WY, Levine JM (1999). "A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus". Neuroscience. 92 (1): 83–95. doi:10.1016/S0306-4522(98)00751-9. PMID 10392832. S2CID 10924179.
- ^ Bergles DE, Jahr CE (December 1997). "Synaptic activation of glutamate transporters in hippocampal astrocytes". Neuron. 19 (6): 1297–308. doi:10.1016/S0896-6273(00)80420-1. PMID 9427252. S2CID 5564226.
- ^ Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE (March 2007). "Vesicular release of glutamate from unmyelinated axons in white matter". Nature Neuroscience. 10 (3): 321–30. doi:10.1038/nn1854. PMC 2140234. PMID 17293857.
- ^ Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M (March 1999). "Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter". Glia. 26 (1): 84–91. doi:10.1002/(SICI)1098-1136(199903)26:1<84::AID-GLIA9>3.0.CO;2-L. PMID 10088675.
- ^ Miller RH (March 1996). "Oligodendrocyte origins". Trends in Neurosciences. 19 (3): 92–6. doi:10.1016/S0166-2236(96)80036-1. PMID 9054062. S2CID 22746971.
- ^ Maki T, Maeda M, Uemura M, Lo EK, Terasaki Y, Liang AC, Shindo A, Choi YK, Taguchi A, Matsuyama T, Takahashi R, Ihara M, Arai K (June 2015). "Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter". Neuroscience Letters. 597: 164–9. doi:10.1016/j.neulet.2015.04.047. PMC 4443478. PMID 25936593.
- ^ Birey F, Aguirre A (April 2015). "Age-Dependent Netrin-1 Signaling Regulates NG2+ Glial Cell Spatial Homeostasis in Normal Adult Gray Matter". The Journal of Neuroscience. 35 (17): 6946–51. doi:10.1523/JNEUROSCI.0356-15.2015. PMC 4412904. PMID 25926469.
- ^ Michalski, JP; Kothary, R (2015). "Oligodendrocytes in a Nutshell". Frontiers in Cellular Neuroscience. 9: 340. doi:10.3389/fncel.2015.00340. PMC 4556025. PMID 26388730.
- ^ Bergles DE, Roberts JD, Somogyi P, Jahr CE (May 2000). "Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus". Nature. 405 (6783): 187–91. Bibcode:2000Natur.405..187B. doi:10.1038/35012083. PMID 10821275. S2CID 4422069.
- ^ Steinhäuser C, Gallo V (August 1996). "News on glutamate receptors in glial cells". Trends in Neurosciences. 19 (8): 339–45. doi:10.1016/0166-2236(96)10043-6. PMID 8843603. S2CID 31596399.
- ^ Orduz D, Maldonado PP, Balia M, Vélez-Fort M, de Sars V, Yanagawa Y, Emiliani V, Angulo MC (April 2015). "Interneurons and oligodendrocyte progenitors form a structured synaptic network in the developing neocortex". eLife. 4. doi:10.7554/eLife.06953. PMC 4432226. PMID 25902404.
- ^ Donna J. Osterhout; Amy Wolven; Rebecca M. Wolf; Marilyn D. Resh & Moses V. Chao (1999). "Morphological Differentiation of Oligodendrocytes Requires Activation of Fyn Tyrosine Kinase". Journal of Cell Biology. 145 (6): 1209–1218. doi:10.1083/jcb.145.6.1209. PMC 2133143. PMID 10366594.
- ^ Spassky N, Olivier C, Cobos I, LeBras B, Goujet-Zalc C, Martínez S, Zalc B, Thomas JL (2001). "The early steps of oligodendrogenesis: insights from the study of the plp lineage in the brain of chicks and rodents". Developmental Neuroscience. 23 (4–5): 318–26. doi:10.1159/000048715. PMID 11756747. S2CID 46878049.
- ^ Jump up to: a b c d Pfeiffer SE, Warrington AE, Bansal R (June 1993). "The oligodendrocyte and its many cellular processes". Trends in Cell Biology. 3 (6): 191–7. doi:10.1016/0962-8924(93)90213-K. PMID 14731493.
- ^ Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD (February 2006). "Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage". Nature Neuroscience. 9 (2): 173–9. doi:10.1038/nn1620. PMC 6328015. PMID 16388308.
- ^ El Waly B, Macchi M, Cayre M, Durbec P (2014). "Oligodendrogenesis in the normal and pathological central nervous system". Frontiers in Neuroscience. 8: 145. doi:10.3389/fnins.2014.00145. PMC 4054666. PMID 24971048.
- ^ Wang H, Rusielewicz T, Tewari A, Leitman EM, Einheber S, Melendez-Vasquez CV (August 2012). "Myosin II is a negative regulator of oligodendrocyte morphological differentiation". Journal of Neuroscience Research. 90 (8): 1547–56. doi:10.1002/jnr.23036. PMC 3370114. PMID 22437915.
- ^ Tripathi RB, Clarke LE, Burzomato V, Kessaris N, Anderson PN, Attwell D, Richardson WD (May 2011). "Dorsally and ventrally derived oligodendrocytes have similar electrical properties but myelinate preferred tracts". The Journal of Neuroscience. 31 (18): 6809–6819. doi:10.1523/JNEUROSCI.6474-10.2011. PMC 4227601. PMID 21543611.
- ^ Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M (October 2008). "Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex". The Journal of Neuroscience. 28 (41): 10434–42. doi:10.1523/JNEUROSCI.2831-08.2008. PMC 6671038. PMID 18842903.
- ^ Káradóttir R, Hamilton NB, Bakiri Y, Attwell D (April 2008). "Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter". Nature Neuroscience. 11 (4): 450–6. doi:10.1038/nn2060. PMC 2615224. PMID 18311136.
- ^ Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A (September 2013). "NG2 cells in white matter but not gray matter proliferate in response to PDGF". The Journal of Neuroscience. 33 (36): 14558–66. doi:10.1523/JNEUROSCI.2001-12.2013. PMC 3761056. PMID 24005306.
- ^ Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A (June 1999). "Subventricular zone astrocytes are neural stem cells in the adult mammalian brain". Cell. 97 (6): 703–16. doi:10.1016/s0092-8674(00)80783-7. PMID 10380923. S2CID 16074660.
- ^ Ortega F, Gascón S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B (June 2013). "Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling". Nature Cell Biology. 15 (6): 602–13. doi:10.1038/ncb2736. PMID 23644466. S2CID 23154014.
- ^ Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A (July 2006). "Origin of oligodendrocytes in the subventricular zone of the adult brain". The Journal of Neuroscience. 26 (30): 7907–18. doi:10.1523/JNEUROSCI.1299-06.2006. PMC 6674207. PMID 16870736.
- ^ Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, Monteiro R, Mummery C, Sommer L, Götz M (January 2008). "Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells". The Journal of Neuroscience. 28 (2): 434–46. doi:10.1523/JNEUROSCI.4374-07.2008. PMC 6670509. PMID 18184786.
- ^ Dessaud E, Ribes V, Balaskas N, Yang LL, Pierani A, Kicheva A, Novitch BG, Briscoe J, Sasai N (June 2010). "Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen sonic hedgehog". PLOS Biology. 8 (6): e1000382. doi:10.1371/journal.pbio.1000382. PMC 2879390. PMID 20532235.
- ^ Kim H, Shin J, Kim S, Poling J, Park HC, Appel B (August 2008). "Notch-regulated oligodendrocyte specification from radial glia in the spinal cord of zebrafish embryos". Developmental Dynamics. 237 (8): 2081–9. doi:10.1002/dvdy.21620. PMC 2646814. PMID 18627107.
- ^ Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH (April 2002). "Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection". Cell. 109 (1): 75–86. CiteSeerX 10.1.1.327.1752. doi:10.1016/s0092-8674(02)00678-5. PMID 11955448. S2CID 1865925.
- ^ Relucio J, Menezes MJ, Miyagoe-Suzuki Y, Takeda S, Colognato H (October 2012). "Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone". Glia. 60 (10): 1451–67. doi:10.1002/glia.22365. PMC 5679225. PMID 22706957.
- ^ Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Mi QS, Xin M, Wang F, Appel B, Lu QR (March 2010). "MicroRNA-mediated control of oligodendrocyte differentiation". Neuron. 65 (5): 612–26. doi:10.1016/j.neuron.2010.02.018. PMC 2855245. PMID 20223198.
- ^ Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W. D. (2005). "Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage". Nature Neuroscience. 9 (2): 173–179. doi:10.1038/nn1620. PMC 6328015. PMID 16388308.
- ^ Nishiyama, A.; Komitova, M.; Suzuki, R.; Zhu, X. (2009). "Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity". Nature Reviews Neuroscience. 10 (1): 9–22. doi:10.1038/nrn2495. PMID 19096367. S2CID 15264205.
- ^ Zhu, X.; Bergles, D. E.; Nishiyama, A. (2007). "NG2 cells generate both oligodendrocytes and gray matter astrocytes". Development. 135 (1): 145–157. doi:10.1242/dev.004895. PMID 18045844.
- ^ Rivers, L. E.; Young, K. M.; Rizzi, M.; Jamen, F. O.; Psachoulia, K.; Wade, A.; Kessaris, N.; Richardson, W. D. (2008). "PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice". Nature Neuroscience. 11 (12): 1392–1401. doi:10.1038/nn.2220. PMC 3842596. PMID 18849983.
- ^ Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Gotz, M. (2008). "Progeny of Olig2-Expressing Progenitors in the Gray and White Matter of the Adult Mouse Cerebral Cortex". Journal of Neuroscience. 28 (41): 10434–10442. doi:10.1523/JNEUROSCI.2831-08.2008. PMC 6671038. PMID 18842903.
- ^ Kang, S. H.; Fukaya, M.; Yang, J. K.; Rothstein, J. D.; Bergles, D. E. (2010). "NG2+ CNS Glial Progenitors Remain Committed to the Oligodendrocyte Lineage in Postnatal Life and following Neurodegeneration". Neuron. 68 (4): 668–681. doi:10.1016/j.neuron.2010.09.009. PMC 2989827. PMID 21092857.
- ^ Richardson, W. D.; Young, K. M.; Tripathi, R. B.; McKenzie, I. (2011). "NG2-glia as Multipotent Neural Stem Cells: Fact or Fantasy?". Neuron. 70 (4): 661–673. doi:10.1016/j.neuron.2011.05.013. PMC 3119948. PMID 21609823.
- ^ Zhu, X.; Zuo, H.; Maher, B. J.; Serwanski, D. R.; Loturco, J. J.; Lu, Q. R.; Nishiyama, A. (2012). "Olig2-dependent developmental fate switch of NG2 cells". Development. 139 (13): 2299–2307. doi:10.1242/dev.078873. PMC 3367441. PMID 22627280.
- ^ Komitova, M.; Zhu, X.; Serwanski, D. R.; Nishiyama, A. (2009). "NG2 cells are distinct from neurogenic cells in the postnatal mouse subventricular zone". The Journal of Comparative Neurology. 512 (5): 702–716. doi:10.1002/cne.21917. PMC 2614367. PMID 19058188.
- ^ Zhu X, Bergles DE, Nishiyama A (January 2008). "NG2 cells generate both oligodendrocytes and gray matter astrocytes". Development. 135 (1): 145–57. doi:10.1242/dev.004895. PMID 18045844.
- ^ Clarke LE, Young KM, Hamilton NB, Li H, Richardson WD, Attwell D (June 2012). "Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse". The Journal of Neuroscience. 32 (24): 8173–85. doi:10.1523/JNEUROSCI.0928-12.2012. PMC 3378033. PMID 22699898.
- ^ Tsoa RW, Coskun V, Ho CK, de Vellis J, Sun YE (May 2014). "Spatiotemporally different origins of NG2 progenitors produce cortical interneurons versus glia in the mammalian forebrain". Proceedings of the National Academy of Sciences of the United States of America. 111 (20): 7444–9. Bibcode:2014PNAS..111.7444T. doi:10.1073/pnas.1400422111. PMC 4034245. PMID 24799701.
- ^ Sakry D, Yigit H, Dimou L, Trotter J (2015). "Oligodendrocyte precursor cells synthesize neuromodulatory factors". PLOS ONE. 10 (5): e0127222. Bibcode:2015PLoSO..1027222S. doi:10.1371/journal.pone.0127222. PMC 4429067. PMID 25966014.
- ^ Jump up to: a b Sakry D, Trotter J (May 2016). "The role of the NG2 proteoglycan in OPC and CNS network function". Brain Research. 1638 (Pt B): 161–166. doi:10.1016/j.brainres.2015.06.003. PMID 26100334. S2CID 32067124.
- ^ Jump up to: a b Sakry D, Neitz A, Singh J, Frischknecht R, Marongiu D, Binamé F, Perera SS, Endres K, Lutz B, Radyushkin K, Trotter J, Mittmann T (November 2014). "Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2". PLOS Biology. 12 (11): e1001993. doi:10.1371/journal.pbio.1001993. PMC 4227637. PMID 25387269.
- ^ Falcao (2018). "Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis". Nature Medicine. 24 (12): 1837–1844. doi:10.1038/s41591-018-0236-y. PMC 6544508. PMID 30420755.
- ^ Bunge MB, Bunge RP, Ris H (May 1961). "Ultrastructural study of remyelination in an experimental lesion in adult cat spinal cord". The Journal of Biophysical and Biochemical Cytology. 10: 67–94. doi:10.1083/jcb.10.1.67. PMC 2225064. PMID 13688845.
- ^ Jump up to: a b Périer O, Grégoire A (December 1965). "Electron microscopic features of multiple sclerosis lesions". Brain. 88 (5): 937–52. doi:10.1093/brain/88.5.937. PMID 5864468.
- ^ Blakemore, W.F. (1974). "Pattern of remyelination in the CNS". Nature. 249 (5457): 577–578. Bibcode:1974Natur.249..577B. doi:10.1038/249577a0. PMID 4834082. S2CID 4246605.
- ^ Smith KJ, Bostock H, Hall SM (April 1982). "Saltatory conduction precedes remyelination in axons demyelinated with lysophosphatidyl choline". Journal of the Neurological Sciences. 54 (1): 13–31. doi:10.1016/0022-510X(82)90215-5. PMID 6804606. S2CID 2748982.
- ^ Albert M, Antel J, Brück W, Stadelmann C (April 2007). "Extensive cortical remyelination in patients with chronic multiple sclerosis". Brain Pathology. 17 (2): 129–38. doi:10.1111/j.1750-3639.2006.00043.x. PMC 8095564. PMID 17388943. S2CID 3158689.
- ^ Jump up to: a b Dawson, M. R.; Polito, A.; Levine, J. M.; Reynolds, R. (2003). "NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS". Molecular and Cellular Neurosciences. 24 (2): 476–488. doi:10.1016/S1044-7431(03)00210-0. PMID 14572468. S2CID 21910392.
- ^ Horner, P. J.; Power, A. E.; Kempermann, G.; Kuhn, H. G.; Palmer, T. D.; Winkler, J.; Thal, L. J.; Gage, F. H. (2000). "Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord". The Journal of Neuroscience. 20 (6): 2218–2228. doi:10.1523/JNEUROSCI.20-06-02218.2000. PMC 6772504. PMID 10704497.
- ^ Gensert, J. M.; Goldman, J. E. (1997). "Endogenous progenitors remyelinate demyelinated axons in the adult CNS". Neuron. 19 (1): 197–203. doi:10.1016/S0896-6273(00)80359-1. PMID 9247275. S2CID 14299146.
- ^ Zawadzka, M.; Rivers, L. E.; Fancy, S. P. J.; Zhao, C.; Tripathi, R.; Jamen, F. O.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; Rowitch, D. H.; Kessaris, N.; Suter, U.; Richardson, W. D.; Franklin, R. J. M. (2010). "CNS-Resident Glial Progenitor/Stem Cells Produce Schwann Cells as well as Oligodendrocytes during Repair of CNS Demyelination". Cell Stem Cell. 6 (6): 578–590. doi:10.1016/j.stem.2010.04.002. PMC 3856868. PMID 20569695.
- ^ Jump up to: a b c McTigue, D. M.; Wei, P.; Stokes, B. T. (2001). "Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord". The Journal of Neuroscience. 21 (10): 3392–3400. doi:10.1523/JNEUROSCI.21-10-03392.2001. PMC 6762495. PMID 11331369.
- ^ Franklin, R. J. M. (2002). "Why does remyelination fail in multiple sclerosis?". Nature Reviews Neuroscience. 3 (9): 705–714. doi:10.1038/nrn917. PMID 12209119. S2CID 19709750.
- ^ Peru, R. L.; Mandrycky, N.; Nait-Oumesmar, B.; Lu, Q. R. (2008). "Paving the Axonal Highway: From Stem Cells to Myelin Repair". Stem Cell Reviews. 4 (4): 304–318. doi:10.1007/s12015-008-9043-z. PMID 18759012. S2CID 19055357.
- ^ Chong, S. Y. C.; Chan, J. R. (2010). "Tapping into the glial reservoir: Cells committed to remaining uncommitted". The Journal of Cell Biology. 188 (3): 305–312. doi:10.1083/jcb.200905111. PMC 2819683. PMID 20142420.
- ^ Prineas, J. W.; Kwon, E. E.; Goldenberg, P. Z.; Ilyas, A. A.; Quarles, R. H.; Benjamins, J. A.; Sprinkle, T. J. (1989). "Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions". Laboratory Investigation. 61 (5): 489–503. PMID 2811298.
- ^ Chang, A.; Tourtellotte, W. W.; Rudick, R.; Trapp, B. D. (2002). "Premyelinating Oligodendrocytes in Chronic Lesions of Multiple Sclerosis". New England Journal of Medicine. 346 (3): 165–173. doi:10.1056/NEJMoa010994. PMID 11796850.
- ^ Zhu, X.; Hill, R. A.; Dietrich, D.; Komitova, M.; Suzuki, R.; Nishiyama, A. (2011). "Age-dependent fate and lineage restriction of single NG2 cells". Development. 138 (4): 745–753. doi:10.1242/dev.047951. PMC 3026417. PMID 21266410.
- ^ Jump up to: a b Butt, A. M.; Duncan, A.; Hornby, M. F.; Kirvell, S. L.; Hunter, A.; Levine, J. M.; Berry, M. (1999). "Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter". Glia. 26 (1): 84–91. doi:10.1002/(SICI)1098-1136(199903)26:1<84::AID-GLIA9>3.0.CO;2-L. PMID 10088675.
- ^ Bergles, D. E.; Roberts, J. D. B.; Somogyi, P.; Jahr, C. E. (2000). "Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus". Nature. 405 (6783): 187–191. Bibcode:2000Natur.405..187B. doi:10.1038/35012083. PMID 10821275. S2CID 4422069.
- ^ Kukley, M.; Capetillo-Zarate, E.; Dietrich, D. (2007). "Vesicular glutamate release from axons in white matter". Nature Neuroscience. 10 (3): 311–320. doi:10.1038/nn1850. PMID 17293860. S2CID 8767161.
- ^ De Biase, L. M.; Nishiyama, A.; Bergles, D. E. (2010). "Excitability and Synaptic Communication within the Oligodendrocyte Lineage". Journal of Neuroscience. 30 (10): 3600–3611. doi:10.1523/JNEUROSCI.6000-09.2010. PMC 2838193. PMID 20219994.
- ^ Kukley, M.; Nishiyama, A.; Dietrich, D. (2010). "The Fate of Synaptic Input to NG2 Glial Cells: Neurons Specifically Downregulate Transmitter Release onto Differentiating Oligodendroglial Cells". Journal of Neuroscience. 30 (24): 8320–8331. doi:10.1523/JNEUROSCI.0854-10.2010. PMC 6634580. PMID 20554883.
- ^ Kukley, M.; Kiladze, M.; Tognatta, R.; Hans, M.; Swandulla, D.; Schramm, J.; Dietrich, D. (2008). "Glial cells are born with synapses". The FASEB Journal. 22 (8): 2957–2969. doi:10.1096/fj.07-090985. PMID 18467596. S2CID 25966213.
- ^ Keirstead HS, Blakemore WF (November 1997). "Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord". Journal of Neuropathology and Experimental Neurology. 56 (11): 1191–201. doi:10.1097/00005072-199711000-00003. PMID 9370229.
- ^ Ffrench-Constant C, Raff MC (1986). "Proliferating bipotential glial progenitor cells in adult rat optic nerve". Nature. 319 (6053): 499–502. Bibcode:1986Natur.319..499F. doi:10.1038/319499a0. PMID 3945333. S2CID 4254924.
- ^ Scolding NJ, Rayner PJ, Sussman J, Shaw C, Compston DA (February 1995). "A proliferative adult human oligodendrocyte progenitor". NeuroReport. 6 (3): 441–5. doi:10.1097/00001756-199502000-00009. PMID 7766839.
- ^ Zhang SC, Ge B, Duncan ID (March 1999). "Adult brain retains the potential to generate oligodendroglial progenitors with extensive myelination capacity". Proceedings of the National Academy of Sciences of the United States of America. 96 (7): 4089–94. Bibcode:1999PNAS...96.4089Z. doi:10.1073/pnas.96.7.4089. PMC 22425. PMID 10097168.
- ^ Luyt K, Varadi A, Halfpenny CA, Scolding NJ, Molnar E (June 2004). "Metabotropic glutamate receptors are expressed in adult human glial progenitor cells". Biochemical and Biophysical Research Communications. 319 (1): 120–9. doi:10.1016/j.bbrc.2004.04.158. PMID 15158450.
- ^ Heese K, Hock C, Otten U (February 1998). "Inflammatory signals induce neurotrophin expression in human microglial cells". Journal of Neurochemistry. 70 (2): 699–707. doi:10.1046/j.1471-4159.1998.70020699.x. PMID 9453564. S2CID 11739236.
- ^ Jump up to: a b Niehaus A, Shi J, Grzenkowski M, Diers-Fenger M, Archelos J, Hartung HP, Toyka K, Brück W, Trotter J (September 2000). "Patients with active relapsing-remitting multiple sclerosis synthesize antibodies recognizing oligodendrocyte progenitor cell surface protein: implications for remyelination". Annals of Neurology. 48 (3): 362–71. doi:10.1002/1531-8249(200009)48:3<362::AID-ANA11>3.0.CO;2-6. PMID 10976643.
- ^ Lakatos A, Franklin RJ, Barnett SC (December 2000). "Olfactory ensheathing cells and Schwann cells differ in their in vitro interactions with astrocytes". Glia. 32 (3): 214–25. doi:10.1002/1098-1136(200012)32:3<214::AID-GLIA20>3.0.CO;2-7. PMID 11102963.
- ^ Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (July 2003). "Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging". Nature Neuroscience. 6 (7): 750–7. doi:10.1038/nn1075. PMID 12808459. S2CID 827480.
- ^ Hirano, M.; Goldman, J. E. (1988). "Gliogenesis in rat spinal cord: Evidence for origin of astrocytes and oligodendrocytes from radial precursors". Journal of Neuroscience Research. 21 (2–4): 155–167. doi:10.1002/jnr.490210208. PMID 3216418. S2CID 43450904.
- ^ Bankir, L.; Bouby, N.; Trinh-Trang-Tan, M. M. (1987). "Heterogeneity of nephron anatomy". Kidney International Supplements. 20: S25–S39. PMID 3298801.
- ^ Raff, M. C.; Miller, R. H.; Noble, M. (1983). "A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium". Nature. 303 (5916): 390–396. Bibcode:1983Natur.303..390R. doi:10.1038/303390a0. PMID 6304520. S2CID 4301091.
- ^ Pringle, N. P.; Mudhar, H. S.; Collarini, E. J.; Richardson, W. D. (1992). "PDGF receptors in the rat CNS: During late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage". Development. 115 (2): 535–551. doi:10.1242/dev.115.2.535. PMID 1425339.
- ^ Jump up to: a b Stallcup, W. B.; Beasley, L.; Levine, J. (1983). "Cell-surface molecules that characterize different stages in the development of cerebellar interneurons". Cold Spring Harbor Symposia on Quantitative Biology. 48 (2): 761–774. doi:10.1101/SQB.1983.048.01.078. PMID 6373111.
- ^ Stallcup, W. B.; Cohn, M. (1976). "Electrical properties of a clonal cell line as determined by measurement of ion fluxes". Experimental Cell Research. 98 (2): 277–284. doi:10.1016/0014-4827(76)90439-0. PMID 943300.
- ^ Wilson, S. S.; Baetge, E. E.; Stallcup, W. B. (1981). "Antisera specific for cell lines with mixed neuronal and glial properties". Developmental Biology. 83 (1): 146–153. doi:10.1016/S0012-1606(81)80017-6. PMID 6263737.
- ^ Shaĭtan, K. V.; Ermolaeva, M. D.; Saraĭkin, S. S. (1999). "Molecular dynamics of oligopeptides. 3. Maps of levels of free energy of modified dipeptides and dynamic correlation in amino acid residues". Biofizika. 44 (1): 18–21. PMID 10330580.
- ^ Nishiyama, A.; Lin, X. -H.; Giese, N.; Heldin, C. -H.; Stallcup, W. B. (1996). "Co-localization of NG2 proteoglycan and PDGF ?-receptor on O2A progenitor cells in the developing rat brain". Journal of Neuroscience Research. 43 (3): 299–314. doi:10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. PMID 8714519.
External links[]
 Media related to Oligodendrocyte progenitor cell at Wikimedia Commons
Media related to Oligodendrocyte progenitor cell at Wikimedia Commons
- Nervous tissue cells
- Glial cells