Organocadmium compound
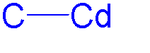
An organocadmium compound is an organometallic compound containing a carbon to cadmium chemical bond. Organocadmium chemistry describes physical properties, synthesis, reactions and use of these compounds.[1] Cadmium shares group 12 with zinc and mercury and their corresponding chemistries have much in common.
One of the simplest examples is dimethylcadmium, which has a C-Cd bond length of 213 pm.[2] All organocadmium compounds are sensitive to air, light, and moisture.
Synthesis[]
The first organocadmium compounds, dimethylcadmium, CH3-Cd-CH3, and diethylcadmium, CH3CH2-Cd-CH2CH3, were prepared in 1917 by Erich Krause. In general, they are prepared by transmetalation or by an exchange reaction between an organometallic reagent and a cadmium salt.[3]
One procedure for the synthesis of diethylcadmium is by the reaction of cadmium bromide with two equivalents of the Grignard reagent ethylmagnesium bromide in diethyl ether. Diethylcadmium is a colorless oil with melting point −21 °C.
Diphenylcadmium can be prepared by the reaction of phenyllithium with cadmium bromide. Diphenylcadmium is a solid with a melting point of 174 °C.
Fluoroalkyl and alkenyl derivatives[]
Following established trends, perfluorinated alkyl and alkenyl derivatives of cadmium exhibit improved thermal stability. The alkenyl derivatives are generated by the addition of iodotrifluoroethylene to cadmium metal.[4]
Reactions[]
The synthetic utility of organocadmiums is limited. They are less nucleophilic than the organozincs. This reduced reactivity is demonstrated in the conversion of acyl chlorides to ketones with these reagents.[5] This reaction was reported by Henry Gilman in 1936 and was used until less toxic cuprates were available. The related Grignard reagent would react further, giving to the tertiary alcohol. Methyl cadmium was used in one of the steps leading to cholesterol total synthesis:[6]
Another example of the synthetic use of an organocadmium is the reaction of diisoamylcadmium with β-carbomethoxypropionyl chloride to methyl 4-keto-7-methyloctanoate without reacting further with the ketone group or the ester group.[7]
This selectivity exists provided that the reaction is carried out salt free.[8] When the cadmium reagent is generated in situ from a cadmium salt, the halide generates a more nucleophilic organocadmium reagent, an ate complex. The same salt effect can be observed with organozinc compounds.
Dimethylcadmium has also used to synthesize colloidal nanocrystals of II-VI materials. Tts toxic and volatile nature has led researchers to look elsewhere for cadmium precursors such as cadmium oxide.[9][10]
Toxicity[]
Cadmium compounds are toxic. Dimethylcadmium is toxic to the kidney, the liver, the central nervous system and the respiratory organs when inhaled.[11] Cadmium compounds in general are considered to be carcinogen to humans by the IARC.[12]
See also[]
- Other chemistries of carbon with other group 12 elements: organozinc compounds and organomercury compounds.
References[]
- ^ Synthetic Methods of Organometallic and Inorganic Chemistry Vol 5, Copper, Silver, Gold, Zinc, Cadmium, and Mercury W.A. Herrmann Ed. ISBN 3-13-103061-5
- ^ Felix Hanke; Sarah Hindley; Anthony C. Jones; Alexander Steiner (2016). "The Solid State Structures of the High and Low Temperature Phases of Dimethylcadmium". Chemical Communications. 52 (66): 10144–10146. doi:10.1039/c6cc05851e. PMID 27457504.
- ^ Erich Krause (1917). "Einfache Cadmiumdialkyle. (I. Mitteilung über organische Cadmium-Verbindungen.)". Berichte der deutschen chemischen Gesellschaft. 50 (2): 1813–1822. doi:10.1002/cber.19170500292.
- ^ Burton, Donald J.; Yang, Zhen-Yu; Morken, Peter A. (1994). "Fluorinated organometallics: Vinyl, Alkynyl, Allyl, Benzyl, Propargyl and Aryl". Tetrahedron. 50 (10): 2993–3063. doi:10.1016/S0040-4020(01)81105-4.
- ^ David A. Shirley (2011). "The Synthesis of Ketones from Acid Halides and Organometallic Compounds of Magnesium, Zinc, and Cadmium". Org. Reactions. doi:10.1002/0471264180.or008.02.
- ^ Woodward, R. B.; Sondheimer, Franz; Taub, David; Heusler, Karl; McLamore, W. M. (1952). "The Total Synthesis of Steroids". Journal of the American Chemical Society. 74 (17): 4223–51. doi:10.1021/ja01137a001.
- ^ Cason, James; Proutyear=1948, Franklin S. "Methyl 4-Keto-7-Methyloctanoate". Organic Syntheses. 28: 75. doi:10.15227/orgsyn.028.0075.
- ^ Jones, Paul R.; Desio, Peter J. (1978). "The less familiar reactions of organocadmium reagents". Chemical Reviews. 78 (5): 491–516. doi:10.1021/cr60315a001.
- ^ Peng ZA, Peng X (2001). "Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor". Journal of the American Chemical Society. 123 (1): 183–4. doi:10.1021/ja003633m. PMID 11273619.
- ^ http://www.cchem.berkeley.edu/~pagrp/index.html[full citation needed]
- ^ Spiridonova EIa (1991). "[Experimental study of toxic properties of dimethylcadmium]". Gigiena Truda I Professional'nye Zabolevaniia (in Russian) (6): 14–7. PMID 1916391.
- ^ http://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C-8.pdf[full citation needed]
- Organometallic compounds
- Cadmium compounds
