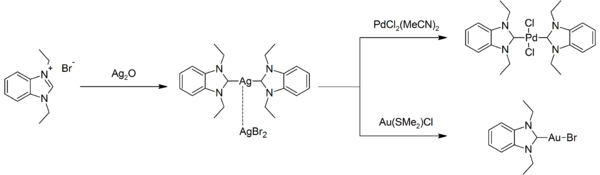Organosilver chemistry

Organosilver chemistry in chemistry of compounds containing a carbon to silver chemical bond.[1] The theme is less developed than organocopper chemistry.
The first attempts in organosilver were recorded by Buckton in 1859[2] and by J. A. Wanklyn & L. Carius in 1861.[3] The synthesis of methyl silver was described by Semerano and Riccoboni[4] Poor thermal stability is reflected in decomposition temperatures of AgMe (-50 °C) versus CuMe (-15 °C) and PhAg (74 °C) vs PhCu (100 °C).
Alkyl, alkenyl, aryl derivatives[]
Phenylsilver can be obtained by reaction of silver nitrate with an trialkylphenyllead or diphenylzinc:[5]
- Ph2Zn + AgNO3 → PhAg + "PhZnNO3"
Like all silver complexes, organosiilver compounds have coordination numbers ≥2. For example, mesitylsilver is a tetramer with 2-coordinate Ag(I) centers. It is produced by reaction of silver chloride and the Grignard reagent:[6]
- AgCl + (CH3)3C6H2MgBr → 1/4 [(CH3)3C6H2Ag]4 + MgClBr
Silver forms complexes with phosphorus ylides such as methylenetriphenylphosphorane:
- AgCl + Ph3P=CH2 → AgCl(Ph3P=CH2)
Alkenylsilver compounds are also more stable than their alkylsilver counterparts. Vinylsilver can be obtained by reaction of silver nitrate with tetravinyllead:[7]
- AgNO3 + (CH2=CH)4Pb → (CH2=CH)Ag + (CH2=CH)3PbNO3
Fluoroalkyl and fluoroalkenyl derivatives[]
Following established trends, perfluorinated alkyl and alkenyl derivatives of silver exhibit significant thermal stability. An alkenyl derivatives are generated by the addition of silver fluoride to hexafluorobutyne and tetrafluoroallene.[8][9]
- AgF + CF2=CF(CF3) → AgCF(CF3)2
Organosilver compounds usually have the oxidation state +1. A notable exception is Ag(CF3)4−.
Carbene and CO complexes[]
Silver forms relatively fragile complexes with CO, including [Ag(CO)n]+ (n = 1, 2, 3).[10]
Silver-NHC complexes are numerous. Some are commonly used to prepare other NHC complexes by displacing labile ligands. For example, the reaction of the bis(NHC)silver(I) complex with bis(acetonitrile)palladium dichloride or chlorido(dimethyl sulfide)gold(I):[11]
Alkene complexes[]

Like other heavy d10 metal ions, Ag+ has a pronounced affinity for alkenes. The ability of silver to form alkene complexes has long been exploited in the separation of alkenes by "argentation chromatography", which uses a support containing silver salts.[13] Illustrative is [Ag(C2H4)3]+.[14]
Catalysis[]
In catalysis silver is active as silver oxide in the Wolff rearrangement. Silver is also present in other carbon-carbon bond skeletal rearrangements such as the quadricyclane to norbornadiene rearrangement, the cubane to cuneane rearrangement and the rearrangement of the cyclobutadiene dimer to cyclooctatetraene.
Further reading[]
- W.A. Herrmann, ed. (1999). Synthetic Methods of Organometallic and Inorganic Chemistry. Vol. 5, Copper, Silver, Gold, Zinc, Cadmium, and Mercury. Stuttgart: Thieme. ISBN 3-13-103061-5.
- Christoph Elschenbroich (2006). Organometallics (3 ed.). Weinheim: Wiley-VCH. ISBN 3-527-29390-6.
- The Chemistry of Organic Derivatives of Gold and Silver. Edited by Saul Patai and Zvi Rappoport Copyright 1999 John Wiley & Sons, Ltd. ISBN 0-471-98164-8
References[]
- ^ Pouwer, Rebecca H.; Williams, Craig M. (2010). "Silver Alkyls, Alkenyls, Aryls, and Alkynyls in Organic Synthesis". Silver in Organic Chemistry. pp. 1–41. doi:10.1002/9780470597521.ch1. ISBN 9780470597521.
- ^ Buckton, G. B. (1859). "Untersuchungen über organische Metallverbindungen". Annalen der Chemie und Pharmacie. 109 (2): 218–227. doi:10.1002/jlac.18591090216.
- ^ Wanklyn, J. A.; Carius, L. (1861). "10. Ueber eine neue Wasserstoffverbindung des Eisens". Annalen der Chemie und Pharmacie. 120 (1): 69. doi:10.1002/jlac.18611200107.
- ^ Semerano, G.; Riccoboni, L. (1941). "Beitrag zur Kenntnis der metallorganischen Verbindungen, I. Mitteil.: Silbermethyl, Silber-äthyl und Silber-n-propyl". Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 74 (7): 1089. doi:10.1002/cber.19410740703.
- ^ Boersma, J; Des Tombe, F.J.A.; Weijers, F.; Van Der Kerk, G.J.M. (1977). "A new, easy synthesis of phenylsilver". J. Organomet. Chem. 124 (2): 229. doi:10.1016/S0022-328X(00)90970-7.
- ^ Meyer, Edouard Marc.; Gambarotta, Sandro.; Floriani, Carlo.; Chiesi-Villa, Angiola.; Guastini, Carlo. (1989). "Polynuclear Aryl Derivatives of Group 11 metals. Synthesis, Solid State-Solution Structural Rrelationship, and Reactivity with Phosphines". Organometallics. 8 (4): 1067–1079. doi:10.1021/om00106a031.
- ^ Holliday, A; Pendlebury, R.E. (1967). "Vinyllead compounds I. Cleavage of vinyl groups from tetravinyllead". J. Organomet. Chem. 7 (2): 281–284. doi:10.1016/S0022-328X(00)91078-7.
- ^ Burton, Donald J.; Yang, Zhen-Yu; Morken, Peter A. (1994). "Fluorinated organometallics: Vinyl, Alkynyl, Allyl, Benzyl, Propargyl and Aryl". Tetrahedron. 50 (10): 2993–3063. doi:10.1016/S0040-4020(01)81105-4.
- ^ Miller, W. T.; Burnard, R. J. (1968). "Perfluoroalkylsilver compounds". J. Am. Chem. Soc. 90 (26): 7367–7368. doi:10.1021/ja01028a047.
- ^ Strauss, Steven H. (2000). "Copper(I) and silver(I) carbonyls. To be or not to be nonclassical". Journal of the Chemical Society, Dalton Transactions: 1–6. doi:10.1039/A908459B.
- ^ Wang, Harrison M. J.; Lin, Ivan J. B. (1998). "Facile Synthesis of Silver(I)−Carbene Complexes. Useful Carbene Transfer Agents". Organometallics. 17 (5): 972. doi:10.1021/om9709704.
- ^ Rencken, Ilmarie; Boeyens, Jan C. A.; Orchard, S. Walter (1988). "Crystal Structures of the trans-Cyclooctene Complexes of Copper(I) Chloride and Silver Nitrate". Journal of Crystallographic and Spectroscopic Research. 18 (3): 293–306. doi:10.1007/BF01194320. S2CID 94984101.
- ^ Boryana Nikolova-Damyanova. "Principles of Silver Ion Complexation with Double Bonds".
- ^ Fianchini, Mauro; Campana, Charles F.; Chilukuri, Bhaskar; Cundari, Thomas R.; Petricek, Vaclav; Dias, H. V. Rasika (2013). "Use of [SbF6]− to Isolate Cationic Copper and Silver Adducts with More than One Ethylene on the Metal Center". Organometallics. 32 (10): 3034–3041. doi:10.1021/om4002439.
- Organosilver compounds