Oxygen toxicity
| Oxygen toxicity | |
|---|---|
| Other names | Oxygen toxicity syndrome, oxygen intoxication, oxygen poisoning |
 | |
| In 1942–43 the UK Government carried out extensive testing for oxygen toxicity in divers. The chamber is pressurised with air to 3.7 bar. The subject in the centre is breathing 100% oxygen from a mask.[1] | |
| Specialty | Emergency medicine |
Oxygen toxicity is a condition resulting from the harmful effects of breathing molecular oxygen (O
2) at increased partial pressures. Severe cases can result in cell damage and death, with effects most often seen in the central nervous system, lungs, and eyes. Historically, the central nervous system condition was called the Paul Bert effect, and the pulmonary condition the Lorrain Smith effect, after the researchers who pioneered the discoveries and descriptions in the late 19th century. Oxygen toxicity is a concern for underwater divers, those on high concentrations of supplemental oxygen (particularly premature babies), and those undergoing hyperbaric oxygen therapy.
The result of breathing increased partial pressures of oxygen is hyperoxia, an excess of oxygen in body tissues. The body is affected in different ways depending on the type of exposure. Central nervous system toxicity is caused by short exposure to high partial pressures of oxygen at greater than atmospheric pressure. Pulmonary and ocular toxicity result from longer exposure to increased oxygen levels at normal pressure. Symptoms may include disorientation, breathing problems, and vision changes such as myopia. Prolonged exposure to above-normal oxygen partial pressures, or shorter exposures to very high partial pressures, can cause oxidative damage to cell membranes, collapse of the alveoli in the lungs, retinal detachment, and seizures. Oxygen toxicity is managed by reducing the exposure to increased oxygen levels. Studies show that, in the long term, a robust recovery from most types of oxygen toxicity is possible.
Protocols for avoidance of the effects of hyperoxia exist in fields where oxygen is breathed at higher-than-normal partial pressures, including underwater diving using compressed breathing gases, hyperbaric medicine, neonatal care and human spaceflight. These protocols have resulted in the increasing rarity of seizures due to oxygen toxicity, with pulmonary and ocular damage being mainly confined to the problems of managing premature infants.
In recent years, oxygen has become available for recreational use in oxygen bars. The US Food and Drug Administration has warned those suffering from problems such as heart or lung disease not to use oxygen bars. Scuba divers use breathing gases containing up to 100% oxygen, and should have specific training in using such gases.
Classification[]

The effects of oxygen toxicity may be classified by the organs affected, producing three principal forms:[2][3][4]
- Central nervous system, characterised by convulsions followed by unconsciousness, occurring under hyperbaric conditions;
- Pulmonary (lungs), characterised by difficulty in breathing and pain within the chest, occurring when breathing increased pressures of oxygen for extended periods;
- Ocular (retinopathic conditions), characterised by alterations to the eyes, occurring when breathing increased pressures of oxygen for extended periods.
Central nervous system oxygen toxicity can cause seizures, brief periods of rigidity followed by convulsions and unconsciousness, and is of concern to divers who encounter greater than atmospheric pressures. Pulmonary oxygen toxicity results in damage to the lungs, causing pain and difficulty in breathing. Oxidative damage to the eye may lead to myopia or partial detachment of the retina. Pulmonary and ocular damage are most likely to occur when supplemental oxygen is administered as part of a treatment, particularly to newborn infants, but are also a concern during hyperbaric oxygen therapy.
Oxidative damage may occur in any cell in the body but the effects on the three most susceptible organs will be the primary concern. It may also be implicated in damage to red blood cells (haemolysis),[5][6] the liver,[7] heart,[8] endocrine glands (adrenal glands, gonads, and thyroid),[9][10][11] or kidneys,[12] and general damage to cells.[2][13]
In unusual circumstances, effects on other tissues may be observed: it is suspected that during spaceflight, high oxygen concentrations may contribute to bone damage.[14] Hyperoxia can also indirectly cause carbon dioxide narcosis in patients with lung ailments such as chronic obstructive pulmonary disease or with central respiratory depression.[14] Hyperventilation of atmospheric air at atmospheric pressures does not cause oxygen toxicity, because sea-level air has a partial pressure of oxygen of 0.21 bar (21 kPa) whereas toxicity does not occur below 0.3 bar (30 kPa).[15]
Signs and symptoms[]
| Exposure (mins.) | Num. of subjects | Symptoms |
|---|---|---|
| 96 | 1 | Prolonged dazzle; severe spasmodic vomiting |
| 60–69 | 3 | Severe lip-twitching; Euphoria; Nausea and vertigo; arm twitch |
| 50–55 | 4 | Severe lip-twitching; Dazzle; Blubbering of lips; fell asleep; Dazed |
| 31–35 | 4 | Nausea, vertigo, lip-twitching; Convulsed |
| 21–30 | 6 | Convulsed; Drowsiness; Severe lip-twitching; epigastric aura; twitch L arm; amnesia |
| 16–20 | 8 | Convulsed; Vertigo and severe lip twitching; epigastric aura; spasmodic respiration; |
| 11–15 | 4 | Inspiratory predominance; lip-twitching and syncope; Nausea and confusion |
| 6–10 | 6 | Dazed and lip-twitching; paraesthesiae; vertigo; "Diaphragmatic spasm"; Severe nausea |
Central nervous system[]
Central nervous system oxygen toxicity manifests as symptoms such as visual changes (especially tunnel vision), ringing in the ears (tinnitus), nausea, twitching (especially of the face), behavioural changes (irritability, anxiety, confusion), and dizziness. This may be followed by a tonic–clonic seizure consisting of two phases: intense muscle contraction occurs for several seconds (tonic phase); followed by rapid spasms of alternate muscle relaxation and contraction producing convulsive jerking (clonic phase). The seizure ends with a period of unconsciousness (the postictal state).[16][17] The onset of seizure depends upon the partial pressure of oxygen in the breathing gas and exposure duration. However, exposure time before onset is unpredictable, as tests have shown a wide variation, both amongst individuals, and in the same individual from day to day.[16][18][19] In addition, many external factors, such as underwater immersion, exposure to cold, and exercise will decrease the time to onset of central nervous system symptoms.[1] Decrease of tolerance is closely linked to retention of carbon dioxide.[20][21][22] Other factors, such as darkness and caffeine, increase tolerance in test animals, but these effects have not been proven in humans.[23][24]
Lungs[]
Pulmonary toxicity symptoms result from an inflammation that starts in the airways leading to the lungs and then spreads into the lungs (tracheobronchial tree). The symptoms appear in the upper chest region (substernal and carinal regions).[25][26][27] This begins as a mild tickle on inhalation and progresses to frequent coughing.[25] If breathing increased partial pressures of oxygen continues, patients experience a mild burning on inhalation along with uncontrollable coughing and occasional shortness of breath (dyspnea).[25] Physical findings related to pulmonary toxicity have included bubbling sounds heard through a stethoscope (bubbling rales), fever, and increased blood flow to the lining of the nose (hyperaemia of the nasal mucosa).[27] X-rays of the lungs show little change in the short term, but extended exposure leads to increasing diffuse shadowing throughout both lungs.[25] Pulmonary function measurements are reduced, as noted by a reduction in the amount of air that the lungs can hold (vital capacity) and changes in expiratory function and lung elasticity.[27][28] Tests in animals have indicated a variation in tolerance similar to that found in central nervous system toxicity, as well as significant variations between species. When the exposure to oxygen above 0.5 bar (50 kPa) is intermittent, it permits the lungs to recover and delays the onset of toxicity.[29]
Eyes[]
In premature babies, signs of damage to the eye (retinopathy of prematurity, or ROP) are observed via an ophthalmoscope as a demarcation between the vascularised and non-vascularised regions of an infant's retina. The degree of this demarcation is used to designate four stages: (I) the demarcation is a line; (II) the demarcation becomes a ridge; (III) growth of new blood vessels occurs around the ridge; (IV) the retina begins to detach from the inner wall of the eye (choroid).[30]
Causes[]
Oxygen toxicity is caused by exposure to oxygen at partial pressures greater than those to which the body is normally exposed. This occurs in three principal settings: underwater diving, hyperbaric oxygen therapy, and the provision of supplemental oxygen, particularly to premature infants. In each case, the risk factors are markedly different.
Central nervous system toxicity[]
Exposures, from minutes to a few hours, to partial pressures of oxygen above 1.6 bars (160 kPa)—about eight times normal atmospheric partial pressure—are usually associated with central nervous system oxygen toxicity and are most likely to occur among patients undergoing hyperbaric oxygen therapy and divers. Since sea level atmospheric pressure is about 1 bar (100 kPa), central nervous system toxicity can only occur under hyperbaric conditions, where ambient pressure is above normal.[31][32] Divers breathing air at depths beyond 60 m (200 ft) face an increasing risk of an oxygen toxicity "hit" (seizure). Divers breathing a gas mixture enriched with oxygen, such as nitrox, can similarly suffer a seizure at shallower depths, should they descend below the maximum operating depth allowed for the mixture.[33]
Lung toxicity[]

The lungs and the remainder of the respiratory tract are exposed to the highest concentration of oxygen in the human body and are therefore the first organs to show toxicity. Pulmonary toxicity occurs only with exposure to partial pressures of oxygen greater than 0.5 bar (50 kPa), corresponding to an oxygen fraction of 50% at normal atmospheric pressure. The earliest signs of pulmonary toxicity begin with evidence of tracheobronchitis, or inflammation of the upper airways, after an asymptomatic period between 4 and 22 hours at greater than 95% oxygen,[34] with some studies suggesting symptoms usually begin after approximately 14 hours at this level of oxygen.[35]
At partial pressures of oxygen of 2 to 3 bar (200 to 300 kPa)—100% oxygen at 2 to 3 times atmospheric pressure—these symptoms may begin as early as 3 hours after exposure to oxygen.[34] Experiments on rats breathing oxygen at pressures between 1 and 3 bars (100 and 300 kPa) suggest that pulmonary manifestations of oxygen toxicity may not be the same for normobaric conditions as they are for hyperbaric conditions.[36] Evidence of decline in lung function as measured by pulmonary function testing can occur as quickly as 24 hours of continuous exposure to 100% oxygen,[35] with evidence of diffuse alveolar damage and the onset of acute respiratory distress syndrome usually occurring after 48 hours on 100% oxygen.[34] Breathing 100% oxygen also eventually leads to collapse of the alveoli (atelectasis), while—at the same partial pressure of oxygen—the presence of significant partial pressures of inert gases, typically nitrogen, will prevent this effect.[37]
Preterm newborns are known to be at higher risk for bronchopulmonary dysplasia with extended exposure to high concentrations of oxygen.[38] Other groups at higher risk for oxygen toxicity are patients on mechanical ventilation with exposure to levels of oxygen greater than 50%, and patients exposed to chemicals that increase risk for oxygen toxicity such the chemotherapeutic agent bleomycin.[35] Therefore, current guidelines for patients on mechanical ventilation in intensive care recommends keeping oxygen concentration less than 60%.[34] Likewise, divers who undergo treatment of decompression sickness are at increased risk of oxygen toxicity as treatment entails exposure to long periods of oxygen breathing under hyperbaric conditions, in addition to any oxygen exposure during the dive.[31]
Ocular toxicity[]
Prolonged exposure to high inspired fractions of oxygen causes damage to the retina.[39][40][41] Damage to the developing eye of infants exposed to high oxygen fraction at normal pressure has a different mechanism and effect from the eye damage experienced by adult divers under hyperbaric conditions.[42][43] Hyperoxia may be a contributing factor for the disorder called retrolental fibroplasia or retinopathy of prematurity (ROP) in infants.[42][44] In preterm infants, the retina is often not fully vascularised. Retinopathy of prematurity occurs when the development of the retinal vasculature is arrested and then proceeds abnormally. Associated with the growth of these new vessels is fibrous tissue (scar tissue) that may contract to cause retinal detachment. Supplemental oxygen exposure, while a risk factor, is not the main risk factor for development of this disease. Restricting supplemental oxygen use does not necessarily reduce the rate of retinopathy of prematurity, and may raise the risk of hypoxia-related systemic complications.[42]
Hyperoxic myopia has occurred in closed circuit oxygen rebreather divers with prolonged exposures.[43][45][46] It also occurs frequently in those undergoing repeated hyperbaric oxygen therapy.[40][47] This is due to an increase in the refractive power of the lens, since axial length and keratometry readings do not reveal a corneal or length basis for a myopic shift.[47][48] It is usually reversible with time.[40][47]
Mechanism[]

The biochemical basis for the toxicity of oxygen is the partial reduction of oxygen by one or two electrons to form reactive oxygen species,[49] which are natural by-products of the normal metabolism of oxygen and have important roles in cell signalling.[50] One species produced by the body, the superoxide anion (O−
2),[51] is possibly involved in iron acquisition.[52] Higher than normal concentrations of oxygen lead to increased levels of reactive oxygen species.[53] Oxygen is necessary for cell metabolism, and the blood supplies it to all parts of the body. When oxygen is breathed at high partial pressures, a hyperoxic condition will rapidly spread, with the most vascularised tissues being most vulnerable. During times of environmental stress, levels of reactive oxygen species can increase dramatically, which can damage cell structures and produce oxidative stress.[19][54]
While all the reaction mechanisms of these species within the body are not yet fully understood,[55] one of the most reactive products of oxidative stress is the hydroxyl radical (·OH), which can initiate a damaging chain reaction of lipid peroxidation in the unsaturated lipids within cell membranes.[56] High concentrations of oxygen also increase the formation of other free radicals, such as nitric oxide, peroxynitrite, and trioxidane, which harm DNA and other biomolecules.[19][57] Although the body has many antioxidant systems such as glutathione that guard against oxidative stress, these systems are eventually overwhelmed at very high concentrations of free oxygen, and the rate of cell damage exceeds the capacity of the systems that prevent or repair it.[58][59][60] Cell damage and cell death then result.[61]
Diagnosis[]
Diagnosis of central nervous system oxygen toxicity in divers prior to seizure is difficult as the symptoms of visual disturbance, ear problems, dizziness, confusion and nausea can be due to many factors common to the underwater environment such as narcosis, congestion and coldness. However, these symptoms may be helpful in diagnosing the first stages of oxygen toxicity in patients undergoing hyperbaric oxygen therapy. In either case, unless there is a prior history of epilepsy or tests indicate hypoglycaemia, a seizure occurring in the setting of breathing oxygen at partial pressures greater than 1.4 bar (140 kPa) suggests a diagnosis of oxygen toxicity.[62]
Diagnosis of bronchopulmonary dysplasia in newborn infants with breathing difficulties is difficult in the first few weeks. However, if the infant's breathing does not improve during this time, blood tests and x-rays may be used to confirm bronchopulmonary dysplasia. In addition, an echocardiogram can help to eliminate other possible causes such as congenital heart defects or pulmonary arterial hypertension.[63]
The diagnosis of retinopathy of prematurity in infants is typically suggested by the clinical setting. Prematurity, low birth weight and a history of oxygen exposure are the principal indicators, while no hereditary factors have been shown to yield a pattern.[64]
Prevention[]

The prevention of oxygen toxicity depends entirely on the setting. Both underwater and in space, proper precautions can eliminate the most pernicious effects. Premature infants commonly require supplemental oxygen to treat complications of preterm birth. In this case prevention of bronchopulmonary dysplasia and retinopathy of prematurity must be carried out without compromising a supply of oxygen adequate to preserve the infant's life.
Underwater[]
Oxygen toxicity is a catastrophic hazard in scuba diving, because a seizure results in near certain death by drowning.[33] The seizure may occur suddenly and with no warning symptoms.[17] The effects are sudden convulsions and unconsciousness, during which victims can lose their regulator and drown.[65][66] One of the advantages of a full-face diving mask is prevention of regulator loss in the event of a seizure. As there is an increased risk of central nervous system oxygen toxicity on deep dives, long dives and dives where oxygen-rich breathing gases are used, divers are taught to calculate a maximum operating depth for oxygen-rich breathing gases, and cylinders containing such mixtures should be clearly marked with that depth.[22][67]
In some diver training courses for these types of diving, divers are taught to plan and monitor what is called the 'oxygen clock' of their dives.[67] This is a notional alarm clock, which ticks more quickly at increased oxygen pressure and is set to activate at the maximum single exposure limit recommended in the National Oceanic and Atmospheric Administration Diving Manual.[22][67] For the following partial pressures of oxygen the limits are: 45 minutes at 1.6 bar (160 kPa), 120 minutes at 1.5 bar (150 kPa), 150 minutes at 1.4 bar (140 kPa), 180 minutes at 1.3 bar (130 kPa) and 210 minutes at 1.2 bar (120 kPa), but it is impossible to predict with any reliability whether or when toxicity symptoms will occur.[68][69] Many nitrox-capable dive computers calculate an oxygen loading and can track it across multiple dives. The aim is to avoid activating the alarm by reducing the partial pressure of oxygen in the breathing gas or by reducing the time spent breathing gas of greater oxygen partial pressure. As the partial pressure of oxygen increases with the fraction of oxygen in the breathing gas and the depth of the dive, the diver obtains more time on the oxygen clock by diving at a shallower depth, by breathing a less oxygen-rich gas, or by shortening the duration of exposure to oxygen-rich gases.[70][71]
Diving below 56 m (184 ft) on air would expose a diver to increasing danger of oxygen toxicity as the partial pressure of oxygen exceeds 1.4 bar (140 kPa), so a gas mixture must be used which contains less than 21% oxygen (a hypoxic mixture). Increasing the proportion of nitrogen is not viable, since it would produce a strongly narcotic mixture. However, helium is not narcotic, and a usable mixture may be blended either by completely replacing nitrogen with helium (the resulting mix is called heliox), or by replacing part of the nitrogen with helium, producing a trimix.[72]
Pulmonary oxygen toxicity is an entirely avoidable event while diving. The limited duration and naturally intermittent nature of most diving makes this a relatively rare (and even then, reversible) complication for divers.[73] Established guidelines enable divers to calculate when they are at risk of pulmonary toxicity.[74][75][76] In saturation diving it can be avoided by limiting the oxygen content of gas in living areas to below 0.4 bar.
Hyperbaric setting[]
The presence of a fever or a history of seizure is a relative contraindication to hyperbaric oxygen treatment.[77] The schedules used for treatment of decompression illness allow for periods of breathing air rather than 100% oxygen (oxygen breaks) to reduce the chance of seizure or lung damage. The U.S. Navy uses treatment tables based on periods alternating between 100% oxygen and air. For example, USN table 6 requires 75 minutes (three periods of 20 minutes oxygen/5 minutes air) at an ambient pressure of 2.8 standard atmospheres (280 kPa), equivalent to a depth of 18 metres (60 ft). This is followed by a slow reduction in pressure to 1.9 atm (190 kPa) over 30 minutes on oxygen. The patient then remains at that pressure for a further 150 minutes, consisting of two periods of 15 minutes air/60 minutes oxygen, before the pressure is reduced to atmospheric over 30 minutes on oxygen.[78]
Vitamin E and selenium were proposed and later rejected as a potential method of protection against pulmonary oxygen toxicity.[79][80][81] There is however some experimental evidence in rats that vitamin E and selenium aid in preventing in vivo lipid peroxidation and free radical damage, and therefore prevent retinal changes following repetitive hyperbaric oxygen exposures.[82]
Normobaric setting[]
Bronchopulmonary dysplasia is reversible in the early stages by use of break periods on lower pressures of oxygen, but it may eventually result in irreversible lung injury if allowed to progress to severe damage. One or two days of exposure without oxygen breaks are needed to cause such damage.[14]
Retinopathy of prematurity is largely preventable by screening. Current guidelines require that all babies of less than 32 weeks gestational age or having a birth weight less than 1.5 kg (3.3 lb) should be screened for retinopathy of prematurity at least every two weeks.[83] The National Cooperative Study in 1954 showed a causal link between supplemental oxygen and retinopathy of prematurity, but subsequent curtailment of supplemental oxygen caused an increase in infant mortality. To balance the risks of hypoxia and retinopathy of prematurity, modern protocols now require monitoring of blood oxygen levels in premature infants receiving oxygen.[84]
Hypobaric setting[]
In low-pressure environments oxygen toxicity may be avoided since the toxicity is caused by high partial pressure of oxygen, not merely by high oxygen fraction. This is illustrated by modern pure oxygen use in spacesuits, which must operate at low pressure (also historically, very high percentage oxygen and lower than normal atmospheric pressure was used in early spacecraft, for example, the Gemini and Apollo spacecraft).[85] In such applications as extra-vehicular activity, high-fraction oxygen is non-toxic, even at breathing mixture fractions approaching 100%, because the oxygen partial pressure is not allowed to chronically exceed 0.3 bar (4.4 psi).[85]
Management[]


During hyperbaric oxygen therapy, the patient will usually breathe 100% oxygen from a mask while inside a hyperbaric chamber pressurised with air to about 2.8 bar (280 kPa). Seizures during the therapy are managed by removing the mask from the patient, thereby dropping the partial pressure of oxygen inspired below 0.6 bar (60 kPa).[17]
A seizure underwater requires that the diver be brought to the surface as soon as practicable. Although for many years the recommendation has been not to raise the diver during the seizure itself, owing to the danger of arterial gas embolism (AGE),[86] there is some evidence that the glottis does not fully obstruct the airway.[87] This has led to the current recommendation by the Diving Committee of the Undersea and Hyperbaric Medical Society that a diver should be raised during the seizure's clonic (convulsive) phase if the regulator is not in the diver's mouth—as the danger of drowning is then greater than that of AGE—but the ascent should be delayed until the end of the clonic phase otherwise.[65] Rescuers ensure that their own safety is not compromised during the convulsive phase. They then ensure that where the victim's air supply is established it is maintained, and carry out a controlled buoyant lift. Lifting an unconscious body is taught by most recreational diver training agencies as an advanced skill, and for professional divers it is a basic skill, as it is one of the primary functions of the standby diver. Upon reaching the surface, emergency services are always contacted as there is a possibility of further complications requiring medical attention.[88] The U.S. Navy has procedures for completing the decompression stops where a recompression chamber is not immediately available.[89]
The occurrence of symptoms of bronchopulmonary dysplasia or acute respiratory distress syndrome is treated by lowering the fraction of oxygen administered, along with a reduction in the periods of exposure and an increase in the break periods where normal air is supplied. Where supplemental oxygen is required for treatment of another disease (particularly in infants), a ventilator may be needed to ensure that the lung tissue remains inflated. Reductions in pressure and exposure will be made progressively, and medications such as bronchodilators and pulmonary surfactants may be used.[90]
Divers manage the risk of pulmonary damage by limiting exposure to levels shown to be generally acceptable by experimental evidence, using a system of accumulated oxygen toxicity units which are based on exposure time at specified partial pressures. In the event of emergency treatment for decompression illness, it may be necessary to exceed normal exposure limits to manage more critical symptoms.[91]
Retinopathy of prematurity may regress spontaneously, but should the disease progress beyond a threshold (defined as five contiguous or eight cumulative hours of stage 3 retinopathy of prematurity), both cryosurgery and laser surgery have been shown to reduce the risk of blindness as an outcome. Where the disease has progressed further, techniques such as scleral buckling and vitrectomy surgery may assist in re-attaching the retina.[92]
Prognosis[]
Although the convulsions caused by central nervous system oxygen toxicity may lead to incidental injury to the victim, it remained uncertain for many years whether damage to the nervous system following the seizure could occur and several studies searched for evidence of such damage. An overview of these studies by Bitterman in 2004 concluded that following removal of breathing gas containing high fractions of oxygen, no long-term neurological damage from the seizure remains.[19][93]
The majority of infants who have survived following an incidence of bronchopulmonary dysplasia will eventually recover near-normal lung function, since lungs continue to grow during the first 5–7 years and the damage caused by bronchopulmonary dysplasia is to some extent reversible (even in adults). However, they are likely to be more susceptible to respiratory infections for the rest of their lives and the severity of later infections is often greater than that in their peers.[94][95]
Retinopathy of prematurity (ROP) in infants frequently regresses without intervention and eyesight may be normal in later years. Where the disease has progressed to the stages requiring surgery, the outcomes are generally good for the treatment of stage 3 ROP, but are much worse for the later stages. Although surgery is usually successful in restoring the anatomy of the eye, damage to the nervous system by the progression of the disease leads to comparatively poorer results in restoring vision. The presence of other complicating diseases also reduces the likelihood of a favourable outcome.[96]
Epidemiology[]
The incidence of central nervous system toxicity among divers has decreased since the Second World War, as protocols have developed to limit exposure and partial pressure of oxygen inspired. In 1947, Donald recommended limiting the depth allowed for breathing pure oxygen to 7.6 m (25 ft), which equates to an oxygen partial pressure of 1.8 bar (180 kPa).[98] Over time this limit has been reduced, until today a limit of 1.4 bar (140 kPa) during a recreational dive and 1.6 bar (160 kPa) during shallow decompression stops is generally recommended.[99] Oxygen toxicity has now become a rare occurrence other than when caused by equipment malfunction and human error. Historically, the U.S. Navy has refined its Navy Diving Manual Tables to reduce oxygen toxicity incidents. Between 1995 and 1999, reports showed 405 surface-supported dives using the helium–oxygen tables; of these, oxygen toxicity symptoms were observed on 6 dives (1.5%). As a result, the U.S. Navy in 2000 modified the schedules and conducted field tests of 150 dives, none of which produced symptoms of oxygen toxicity. Revised tables were published in 2001.[100]
The variability in tolerance and other variable factors such as workload have resulted in the U.S. Navy abandoning screening for oxygen tolerance. Of the 6,250 oxygen-tolerance tests performed between 1976 and 1997, only 6 episodes of oxygen toxicity were observed (0.1%).[101][102]
Central nervous system oxygen toxicity among patients undergoing hyperbaric oxygen therapy is rare, and is influenced by a number of a factors: individual sensitivity and treatment protocol; and probably therapy indication and equipment used. A study by Welslau in 1996 reported 16 incidents out of a population of 107,264 patients (0.015%), while Hampson and Atik in 2003 found a rate of 0.03%.[103][104] Yildiz, Ay and Qyrdedi, in a summary of 36,500 patient treatments between 1996 and 2003, reported only 3 oxygen toxicity incidents, giving a rate of 0.008%.[103] A later review of over 80,000 patient treatments revealed an even lower rate: 0.0024%. The reduction in incidence may be partly due to use of a mask (rather than a hood) to deliver oxygen.[105]
Bronchopulmonary dysplasia is among the most common complications of prematurely born infants and its incidence has grown as the survival of extremely premature infants has increased. Nevertheless, the severity has decreased as better management of supplemental oxygen has resulted in the disease now being related mainly to factors other than hyperoxia.[38]
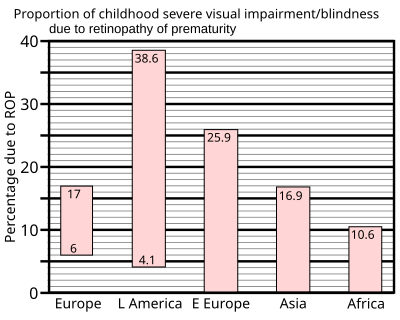
In 1997 a summary of studies of neonatal intensive care units in industrialised countries showed that up to 60% of low birth weight babies developed retinopathy of prematurity, which rose to 72% in extremely low birth weight babies, defined as less than 1 kg (2.2 lb) at birth. However, severe outcomes are much less frequent: for very low birth weight babies—those less than 1.5 kg (3.3 lb) at birth—the incidence of blindness was found to be no more than 8%.[97]
History[]

Central nervous system toxicity was first described by Paul Bert in 1878.[106][107] He showed that oxygen was toxic to insects, arachnids, myriapods, molluscs, earthworms, fungi, germinating seeds, birds, and other animals. Central nervous system toxicity may be referred to as the "Paul Bert effect".[14]
Pulmonary oxygen toxicity was first described by J. Lorrain Smith in 1899 when he noted central nervous system toxicity and discovered in experiments in mice and birds that 0.43 bar (43 kPa) had no effect but 0.75 bar (75 kPa) of oxygen was a pulmonary irritant.[29] Pulmonary toxicity may be referred to as the "Lorrain Smith effect".[14] The first recorded human exposure was undertaken in 1910 by Bornstein when two men breathed oxygen at 2.8 bar (280 kPa) for 30 minutes, while he went on to 48 minutes with no symptoms. In 1912, Bornstein developed cramps in his hands and legs while breathing oxygen at 2.8 bar (280 kPa) for 51 minutes.[3] Smith then went on to show that intermittent exposure to a breathing gas with less oxygen permitted the lungs to recover and delayed the onset of pulmonary toxicity.[29]
Albert R. Behnke et al. in 1935 were the first to observe visual field contraction (tunnel vision) on dives between 1.0 bar (100 kPa) and 4.1 bar (410 kPa).[108][109] During World War II, Donald and Yarbrough et al. performed over 2,000 experiments on oxygen toxicity to support the initial use of closed circuit oxygen rebreathers.[39][110] Naval divers in the early years of oxygen rebreather diving developed a mythology about a monster called "Oxygen Pete", who lurked in the bottom of the Admiralty Experimental Diving Unit "wet pot" (a water-filled hyperbaric chamber) to catch unwary divers. They called having an oxygen toxicity attack "getting a Pete".[111][112]
In the decade following World War II, Lambertsen et al. made further discoveries on the effects of breathing oxygen under pressure and methods of prevention.[113][114] Their work on intermittent exposures for extension of oxygen tolerance and on a model for prediction of pulmonary oxygen toxicity based on pulmonary function are key documents in the development of standard operating procedures when breathing increased pressures of oxygen.[115] Lambertsen's work showing the effect of carbon dioxide in decreasing time to onset of central nervous system symptoms has influenced work from current exposure guidelines to future breathing apparatus design.[21][22][116]
Retinopathy of prematurity was not observed before World War II, but with the availability of supplemental oxygen in the decade following, it rapidly became one of the principal causes of infant blindness in developed countries. By 1960 the use of oxygen had become identified as a risk factor and its administration restricted. The resulting fall in retinopathy of prematurity was accompanied by a rise in infant mortality and hypoxia-related complications. Since then, more sophisticated monitoring and diagnosis have established protocols for oxygen use which aim to balance between hypoxic conditions and problems of retinopathy of prematurity.[97]
Bronchopulmonary dysplasia was first described by Northway in 1967, who outlined the conditions that would lead to the diagnosis.[117] This was later expanded by Bancalari and in 1988 by Shennan, who suggested the need for supplemental oxygen at 36 weeks could predict long-term outcomes.[118] Nevertheless, Palta et al. in 1998 concluded that radiographic evidence was the most accurate predictor of long-term effects.[119]
Bitterman et al. in 1986 and 1995 showed that darkness and caffeine would delay the onset of changes to brain electrical activity in rats.[23][24] In the years since, research on central nervous system toxicity has centred on methods of prevention and safe extension of tolerance.[120] Sensitivity to central nervous system oxygen toxicity has been shown to be affected by factors such as circadian rhythm, drugs, age, and gender.[121][122][123][124] In 1988, Hamilton et al. wrote procedures for the National Oceanic and Atmospheric Administration to establish oxygen exposure limits for habitat operations.[74][75][76] Even today, models for the prediction of pulmonary oxygen toxicity do not explain all the results of exposure to high partial pressures of oxygen.[125]
Society and culture[]
Recreational scuba divers commonly breathe nitrox containing up to 40% oxygen, while technical divers use pure oxygen or nitrox containing up to 80% oxygen. Divers who breathe oxygen fractions greater than of air (21%) need to be trained in the dangers of oxygen toxicity and how to prevent them.[67] In order to buy nitrox, a diver has to show evidence of such qualification.[126]
Since the late 1990s the recreational use of oxygen has been promoted by oxygen bars, where customers breathe oxygen through a nasal cannula. Claims have been made that this reduces stress, increases energy, and lessens the effects of hangovers and headaches, despite the lack of any scientific evidence to support them.[127] There are also devices on sale that offer "oxygen massage" and "oxygen detoxification" with claims of removing body toxins and reducing body fat.[128] The American Lung Association has stated "there is no evidence that oxygen at the low flow levels used in bars can be dangerous to a normal person's health", but the U.S. Center for Drug Evaluation and Research cautions that people with heart or lung disease need their supplementary oxygen carefully regulated and should not use oxygen bars.[127]
Victorian society had a fascination for the rapidly expanding field of science. In "Dr. Ox's Experiment", a short story written by Jules Verne in 1872, the eponymous doctor uses electrolysis of water to separate oxygen and hydrogen. He then pumps the pure oxygen throughout the town of Quiquendone, causing the normally tranquil inhabitants and their animals to become aggressive and plants to grow rapidly. An explosion of the hydrogen and oxygen in Dr Ox's factory brings his experiment to an end. Verne summarised his story by explaining that the effects of oxygen described in the tale were his own invention.[129] There is also a brief episode of oxygen intoxication in his "From the Earth to the Moon".[130]
See also[]
- Effect of oxygen on chronic obstructive pulmonary disease
- Nitrogen narcosis – Reversible narcotic effects of respiratory nitrogen at elevated partial pressures
References[]
- ^ a b c Donald, Part I 1947.
- ^ a b Clark & Thom 2003, pp. 358–60.
- ^ a b Acott, Chris (1999). "Oxygen toxicity: A brief history of oxygen in diving". South Pacific Underwater Medicine Society Journal. 29 (3): 150–55. ISSN 0813-1988. OCLC 16986801. Retrieved 29 April 2008.
- ^ Beehler, CC (1964). "Oxygen and the eye". Survey of Ophthalmology. 9: 549–60. PMID 14232720.
- ^ Goldstein, JR; Mengel, CE (1969). "Hemolysis in mice exposed to varying levels of hyperoxia". Aerospace Medicine. 40 (1): 12–13. PMID 5782651.
- ^ Larkin, EC; Adams, JD; Williams, WT; Duncan, DM (1972). "Hematologic responses to hypobaric hyperoxia". American Journal of Physiology. 223 (2): 431–37. doi:10.1152/ajplegacy.1972.223.2.431. PMID 4403030.
- ^ Schaffner, Fenton; Felig, Philip (1965). "Changes in Hepatic Structure in Rats Produced by Breathing Pure Oxygen". Journal of Cell Biology. 27 (3): 505–17. doi:10.1083/jcb.27.3.505. PMC 2106769. PMID 5885427.
- ^ Caulfield, JB; Shelton, RW; Burke, JF (1972). "Cytotoxic effects of oxygen on striated muscle". Archives of Pathology. 94 (2): 127–32. PMID 5046798.
- ^ Bean, JW; Johnson, PC (1954). "Adrenocortical response to single and repeated exposure to oxygen at high pressure". American Journal of Physiology. 179 (3): 410–44. doi:10.1152/ajplegacy.1954.179.3.410. PMID 13228600.
- ^ Edstrom, JE; Rockert, H (1962). "The effect of oxygen at high pressure on the histology of the central nervous system and sympathetic and endocrine cells". Acta Physiologica Scandinavica. 55 (2–3): 255–63. doi:10.1111/j.1748-1716.1962.tb02438.x. PMID 13889254.
- ^ Gersh, I; Wagner, CE (1945). "Metabolic factors in oxygen poisoning". American Journal of Physiology. 144 (2): 270–77. doi:10.1152/ajplegacy.1945.144.2.270.
- ^ Hess, RT; Menzel, DB (1971). "Effect of dietary antioxidant level and oxygen exposure on the fine structure of the proximal convoluted tubules". Aerospace Medicine. 42 (6): 646–49. PMID 5155150.
- ^
Clark, John M (1974). "The toxicity of oxygen". American Review of Respiratory Disease. 110 (6 Pt 2): 40–50. doi:10.1164/arrd.1974.110.6P2.40 (inactive 31 October 2021). PMID 4613232.
{{cite journal}}: CS1 maint: DOI inactive as of October 2021 (link) (subscription required) - ^ a b c d e Patel, Dharmeshkumar N; Goel, Ashish; Agarwal, SB; Garg, Praveenkumar; Lakhani, Krishna K (2003). "Oxygen toxicity" (PDF). Journal, Indian Academy of Clinical Medicine. 4 (3): 234–37. Retrieved 28 September 2008.
- ^ Clark & Lambertsen 1970, p. 159.
- ^ a b Clark & Thom 2003, p. 376.
- ^ a b c d Bitterman, N (2004). "CNS oxygen toxicity". Undersea and Hyperbaric Medicine. 31 (1): 63–72. PMID 15233161. Retrieved 29 April 2008.
- ^ Lang 2001, p. 82.
- ^ a b Richardson, Drew; Menduno, Michael; Shreeves, Karl, eds. (1996). "Proceedings of rebreather forum 2.0". Diving Science and Technology Workshop: 286. Retrieved 20 September 2008.
- ^ a b c d Richardson, Drew; Shreeves, Karl (1996). "The PADI enriched air diver course and DSAT oxygen exposure limits". South Pacific Underwater Medicine Society Journal. 26 (3). ISSN 0813-1988. OCLC 16986801. Retrieved 2 May 2008.
- ^ a b Bitterman, N; Melamed, Y; Perlman, I (1986). "CNS oxygen toxicity in the rat: role of ambient illumination". Undersea Biomedical Research. 13 (1): 19–25. PMID 3705247. Retrieved 20 September 2008.
- ^ a b Bitterman, N; Schaal, S (1995). "Caffeine attenuates CNS oxygen toxicity in rats". Brain Research. 696 (1–2): 250–53. doi:10.1016/0006-8993(95)00820-G. PMID 8574677. S2CID 9020944.
- ^ a b c d Clark & Thom 2003, p. 383.
- ^ Clark, John M; Lambertsen, Christian J (1971). "Pulmonary oxygen toxicity: a review". Pharmacological Reviews. 23 (2): 37–133. PMID 4948324.
- ^ a b c Clark, John M; Lambertsen, Christian J (1971). "Rate of development of pulmonary O2 toxicity in man during O2 breathing at 2.0 Ata". Journal of Applied Physiology. 30 (5): 739–52. doi:10.1152/jappl.1971.30.5.739. PMID 4929472.
- ^ Clark & Thom 2003, pp. 386–87.
- ^ a b c Smith, J Lorrain (1899). "The pathological effects due to increase of oxygen tension in the air breathed". Journal of Physiology. London: The Physiological Society and Blackwell Publishing. 24 (1): 19–35. doi:10.1113/jphysiol.1899.sp000746. PMC 1516623. PMID 16992479. Note: 1 atmosphere (atm) is 1.013 bars.
- ^ Fielder, Alistair R (1993). Fielder, Alistair R; Best, Anthony; Bax, Martin C O (eds.). The Management of Visual Impairment in Childhood. London: Mac Keith Press : Distributed by Cambridge University Press. p. 33. ISBN 0-521-45150-7.
- ^ a b Smerz, RW (2004). "Incidence of oxygen toxicity during the treatment of dysbarism". Undersea and Hyperbaric Medicine. 31 (2): 199–202. PMID 15485081. Retrieved 30 April 2008.
- ^ Hampson, Neal B; Simonson, Steven G; Kramer, CC; Piantadosi, Claude A (1996). "Central nervous system oxygen toxicity during hyperbaric treatment of patients with carbon monoxide poisoning". Undersea and Hyperbaric Medicine. 23 (4): 215–19. PMID 8989851. Retrieved 29 April 2008.
- ^ a b Lang 2001, p. 7.
- ^ a b c d Bitterman, H (2009). "Bench-to-bedside review: Oxygen as a drug". Critical Care. 13 (1): 205. doi:10.1186/cc7151. PMC 2688103. PMID 19291278.
- ^ a b c Jackson, RM (1985). "Pulmonary oxygen toxicity". Chest. 88 (6): 900–05. doi:10.1378/chest.88.6.900. PMID 3905287.
- ^ Demchenko, Ivan T; Welty-Wolf, Karen E; Allen, Barry W; Piantadosi, Claude A (2007). "Similar but not the same: normobaric and hyperbaric pulmonary oxygen toxicity, the role of nitric oxide". American Journal of Physiology. Lung Cellular and Molecular Physiology. 293 (1): L229–38. doi:10.1152/ajplung.00450.2006. PMID 17416738.
- ^ Wittner, M; Rosenbaum, RM (1966). Pathophysiology of pulmonary oxygen toxicity. Proceedings of the Third International Conference on Hyperbaric Medicine. NAS/NRC, 1404, Washington DC. pp. 179–88. – and others as discussed by Clark & Lambertsen 1970, pp. 256–60
- ^ a b Bancalari, Eduardo; Claure, Nelson; Sosenko, Ilene RS (2003). "Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition". Seminars in Neonatology. London: Elsevier Science. 8 (1): 63–71. doi:10.1016/S1084-2756(02)00192-6. PMID 12667831.
- ^ a b Yarbrough, OD; Welham, W; Brinton, ES; Behnke, Alfred R (1947). "Symptoms of Oxygen Poisoning and Limits of Tolerance at Rest and at Work". Navy Experimental Diving Unit Technical Report 47-01. United States Navy Experimental Diving Unit Technical Report. Retrieved 29 April 2008.
- ^ a b c Anderson, B; Farmer, Joseph C (1978). "Hyperoxic myopia". Transactions of the American Ophthalmological Society. 76: 116–24. PMC 1311617. PMID 754368.
- ^ Ricci, B; Lepore, D; Iossa, M; Santo, A; D'Urso, M; Maggiano, N (1990). "Effect of light on oxygen-induced retinopathy in the rat model. Light and OIR in the rat". Documenta Ophthalmologica. 74 (4): 287–301. doi:10.1007/BF00145813. PMID 1701697. S2CID 688116.
- ^ a b c Drack, AV (1998). "Preventing blindness in premature infants". New England Journal of Medicine. 338 (22): 1620–21. doi:10.1056/NEJM199805283382210. PMID 9603802.
- ^ a b Butler, Frank K; White, E; Twa, M (1999). "Hyperoxic myopia in a closed-circuit mixed-gas scuba diver". Undersea and Hyperbaric Medicine. 26 (1): 41–45. PMID 10353183. Retrieved 29 April 2009.
- ^ Nichols, CW; Lambertsen, Christian (1969). "Effects of high oxygen pressures on the eye". New England Journal of Medicine. 281 (1): 25–30. doi:10.1056/NEJM196907032810106. PMID 4891642.
- ^ Shykoff, Barbara E (2005). "Repeated Six-Hour Dives 1.35 ATM Oxygen Partial Pressure". Nedu-Tr-05-20. Panama City, FL: US Navy Experimental Diving Unit Technical Report. Retrieved 19 September 2008.
- ^ Shykoff, Barbara E (2008). "Pulmonary effects of submerged oxygen breathing in resting divers: repeated exposures to 140 kPa". Undersea and Hyperbaric Medicine. 35 (2): 131–43. PMID 18500077.
- ^ a b c Anderson Jr, B; Shelton, DL (1987). "Axial length in hyperoxic myopia". In: Bove, Alfred A; Bachrach, Arthur J; Greenbaum, Leon (Eds.) Ninth International Symposium of the UHMS. Undersea and Hyperbaric Medical Society: 607–11.
- ^ Schaal, S; Beiran, I; Rubinstein, I; Miller, B; Dovrat, A (2005). "Oxygen effect on ocular lens". Harefuah (in Hebrew). 144 (11): 777–80, 822. PMID 16358652.
- ^ Clark & Thom 2003, p. 360.
- ^ Rhee, SG (2006). "Cell signaling. H2O2, a necessary evil for cell signaling". Science. 312 (5782): 1882–83. doi:10.1126/science.1130481. PMID 16809515. S2CID 83598498.
- ^ Thom, Steven R (1992). "Inert gas enhancement of superoxide radical production". Archives of Biochemistry and Biophysics. 295 (2): 391–96. doi:10.1016/0003-9861(92)90532-2. PMID 1316738.
- ^ Ghio, Andrew J; Nozik-Grayck, Eva; Turi, Jennifer; Jaspers, Ilona; Mercatante, Danielle R; Kole, Ryszard; Piantadosi, Claude A (2003). "Superoxide-dependent iron uptake: a new role for anion exchange protein 2". American Journal of Respiratory Cell and Molecular Biology. 29 (6): 653–60. doi:10.1165/rcmb.2003-0070OC. PMID 12791678.
- ^ Fridovich, I (1998). "Oxygen toxicity: a radical explanation" (PDF). Journal of Experimental Biology. 201 (8): 1203–09. doi:10.1242/jeb.201.8.1203. PMID 9510531.
- ^ Piantadosi, Claude A (2008). "Carbon Monoxide, Reactive Oxygen Signaling, and Oxidative Stress". Free Radical Biology & Medicine. 45 (5): 562–69. doi:10.1016/j.freeradbiomed.2008.05.013. PMC 2570053. PMID 18549826.
- ^ Imlay, JA (2003). "Pathways of oxidative damage". Annual Review of Microbiology. 57: 395–418. doi:10.1146/annurev.micro.57.030502.090938. PMID 14527285.
- ^ Bowen, R. "Free Radicals and Reactive Oxygen". Colorado State University. Archived from the original on 12 May 2008. Retrieved 26 September 2008.
- ^ Oury, TD; Ho, YS; Piantadosi, Claude A; Crapo, JD (1992). "Extracellular superoxide dismutase, nitric oxide, and central nervous system O2 toxicity". Proceedings of the National Academy of Sciences of the United States of America. 89 (20): 9715–19. Bibcode:1992PNAS...89.9715O. doi:10.1073/pnas.89.20.9715. PMC 50203. PMID 1329105.
- ^ Thom, Steven R; Marquis, RE (1987). "Free radical reactions and the inhibitory and lethal actions of high-pressure gases". Undersea Biomedical Research. 14 (6): 485–501. PMID 2825395. Retrieved 26 September 2008.
- ^ Djurhuus, R; Svardal, AM; Thorsen, E (1999). "Glutathione in the cellular defense of human lung cells exposed to hyperoxia and high pressure". Undersea and Hyperbaric Medicine. 26 (2): 75–85. PMID 10372426. Retrieved 26 September 2008.
- ^ Freiberger, John J; Coulombe, Kathy; Suliman, Hagir; Carraway, Martha-sue; Piantadosi, Claude A (2004). "Superoxide dismutase responds to hyperoxia in rat hippocampus". Undersea and Hyperbaric Medicine. 31 (2): 227–32. PMID 15485085. Retrieved 26 September 2008.
- ^ Kim, YS; Kim, SU (1991). "Oligodendroglial cell death induced by oxygen radicals and its protection by catalase". Journal of Neuroscience Research. 29 (1): 100–06. doi:10.1002/jnr.490290111. PMID 1886163. S2CID 19165217.
- ^ NBDHMT (4 February 2009). "Recommended Guidelines for Clinical Internship in Hyperbaric Technology (V: C.D)". Harvey, LA: National Board of Diving and Hyperbaric Medical Technology. Archived from the original on 20 September 2007. Retrieved 26 March 2009.
- ^ "How is bronchopulmonary dysplasia diagnosed?". U.S. Department of Health & Human Services. Retrieved 28 September 2008.
- ^ Regillo, Brown & Flynn 1998, p. 178.
- ^ a b Mitchell, Simon J; Bennett, Michael H; Bird, Nick; Doolette, David J; Hobbs, Gene W; Kay, Edward; Moon, Richard E; Neuman, Tom S; Vann, Richard D; Walker, Richard; Wyatt, HA (2012). "Recommendations for rescue of a submerged unresponsive compressed-gas diver". Undersea & Hyperbaric Medicine. 39 (6): 1099–108. PMID 23342767. Retrieved 13 March 2013.
- ^ Clark & Thom 2003, p. 375.
- ^ a b c d Lang 2001, p. 195.
- ^ Butler, Frank K; Thalmann; Edward D (1986). "Central nervous system oxygen toxicity in closed circuit scuba divers II". Undersea Biomedical Research. 13 (2): 193–223. PMID 3727183. Retrieved 29 April 2008.
- ^ Butler, Frank K (2004). "Closed-circuit oxygen diving in the U.S. Navy". Undersea and Hyperbaric Medicine. 31 (1): 3–20. PMID 15233156. Retrieved 29 April 2008.
- ^ Clark & Lambertsen 1970, pp. 157–62.
- ^ Baker, Erik C (2000). "Oxygen toxicity calculations" (PDF). Retrieved 29 June 2009.
- ^ Hamilton & Thalmann 2003, pp. 475, 479.
- ^ Clark & Lambertsen 1970, p. 270.
- ^ a b Hamilton, RW; Kenyon, David J; Peterson, RE; Butler, GJ; Beers, DM (1988). "Repex habitat diving procedures: Repetitive vertical excursions, oxygen limits, and surfacing techniques". Technical Report 88-1A. Rockville, MD: NOAA Office of Undersea Research. Retrieved 29 April 2008.
- ^ a b Hamilton, Robert W; Kenyon, David J; Peterson, RE (1988). "Repex habitat diving procedures: Repetitive vertical excursions, oxygen limits, and surfacing techniques". Technical Report 88-1B. Rockville, MD: NOAA Office of Undersea Research. Retrieved 29 April 2008.
- ^ a b Hamilton, Robert W (1997). "Tolerating oxygen exposure". South Pacific Underwater Medicine Society Journal. 27 (1). ISSN 0813-1988. OCLC 16986801. Retrieved 29 April 2008.
- ^ Latham, Emi (7 November 2008). "Hyperbaric Oxygen Therapy: Contraindications". Medscape. Retrieved 25 September 2008.
- ^ Schatte, CL (1977). "Dietary selenium and vitamin E as a possible prophylactic to pulmonary oxygen poisoning". Proceedings of the Sixth International Congress on Hyperbaric Medicine, University of Aberdeen, Aberdeen, Scotland. Aberdeen: Aberdeen University Press: 84–91. ISBN 0-08-024918-3. OCLC 16428246.
- ^ Boadi, WY; Thaire, L; Kerem, D; Yannai, S (1991). "Effects of dietary supplementation with vitamin E, riboflavin and selenium on central nervous system oxygen toxicity". Pharmacology & Toxicology. 68 (2): 77–82. doi:10.1111/j.1600-0773.1991.tb02039.x. PMID 1852722.
- ^ Piantadosi, Claude A (2006). In: The Mysterious Malady: Toward an understanding of decompression injuries (DVD). Global Underwater Explorers. Retrieved 2 April 2012.
- ^ Stone, WL; Henderson, RA; Howard, GH; Hollis, AL; Payne, PH; Scott, RL (1989). "The role of antioxidant nutrients in preventing hyperbaric oxygen damage to the retina". Free Radical Biology & Medicine. 6 (5): 505–12. doi:10.1016/0891-5849(89)90043-9. PMID 2744583.
- ^ "UK Retinopathy of Prematurity Guideline" (PDF). Royal College of Paediatrics and Child Health, Royal College of Ophthalmologists & British Association of Perinatal Medicine. 2007. p. i. Archived from the original (PDF) on 18 February 2012. Retrieved 2 April 2009.
- ^ Silverman, William (1980). Retrolental Fibroplasia: A Modern Parable. Grune & Stratton. pp. 39, 41, 143. ISBN 978-0-8089-1264-4.
- ^ a b Webb, James T; Olson, RM; Krutz, RW; Dixon, G; Barnicott, PT (1989). "Human tolerance to 100% oxygen at 9.5 psia during five daily simulated 8-hour EVA exposures". Aviation, Space, and Environmental Medicine. 60 (5): 415–21. doi:10.4271/881071. PMID 2730484.
- ^ Mitchell, Simon J (20 January 2008). "Standardizing CCR rescue skills". RebreatherWorld. Archived from the original on 3 March 2012. Retrieved 26 May 2009. This forum post's author chairs the diving committee of the Undersea and Hyperbaric Medical Society.
- ^ Thalmann, Edward D (2 December 2003). "OXTOX: If You Dive Nitrox You Should Know About OXTOX". Divers Alert Network. Retrieved 11 October 2015. – Section "What do you do if oxygen toxicity or a convulsion happens?"
- ^ "NIH MedlinePlus: Bronchopulmonary dysplasia". U.S. National Library of Medicine. Retrieved 2 October 2008.
- ^ NOAA Diving Program (U.S.) (2001). Joiner, James T. (ed.). NOAA Diving Manual, Diving for Science and Technology (4th ed.). Silver Spring, Maryland: National Oceanic and Atmospheric Administration, Office of Oceanic and Atmospheric Research, National Undersea Research Program. ISBN 978-0-941332-70-5.
- ^ Regillo, Brown & Flynn 1998, p. 184.
- ^ Lambertsen, Christian J (1965). Fenn, WO; Rahn, H (eds.). "Effects of oxygen at high partial pressure". Handbook of Physiology: Respiration. American Physiological Society. Sec 3 Vol 2: 1027–46.
- ^ "National Institutes of Health: What is bronchopulmonary dysplasia?". U.S. Department of Health & Human Services. Retrieved 2 October 2008.
- ^ Spear, Michael L – reviewer (June 2008). "Bronchopulmonary dysplasia (BPD)". Nemours Foundation. Retrieved 3 October 2008.
- ^ Regillo, Brown & Flynn 1998, p. 190.
- ^ a b c Gilbert, Clare (1997). "Retinopathy of prematurity: epidemiology". Journal of Community Eye Health. London: International Centre for Eye Health. 10 (22): 22–24.
- ^ Donald, Part II 1947.
- ^ Lang 2001, p. 183.
- ^ Gerth, Wayne A (2006). "Decompression sickness and oxygen toxicity in U.S. Navy surface-supplied He-O2 diving". Proceedings of Advanced Scientific Diving Workshop. Smithsonian Institution. Retrieved 2 October 2008.
- ^ Walters, KC; Gould, MT; Bachrach, EA; Butler, Frank K (2000). "Screening for oxygen sensitivity in U.S. Navy combat swimmers". Undersea and Hyperbaric Medicine. 27 (1): 21–26. PMID 10813436. Retrieved 2 October 2008.
- ^ Butler, Frank K; Knafelc, ME (1986). "Screening for oxygen intolerance in U.S. Navy divers". Undersea Biomedical Research. 13 (1): 91–98. PMID 3705251. Retrieved 2 October 2008.
- ^ a b Yildiz, S; Ay, H; Qyrdedi, T (2004). "Central nervous system oxygen toxicity during routine hyperbaric oxygen therapy". Undersea and Hyperbaric Medicine. Undersea and Hyperbaric Medical Society, Inc. 31 (2): 189–90. PMID 15485078. Retrieved 3 October 2008.
- ^ Hampson, Neal; Atik, D (2003). "Central nervous system oxygen toxicity during routine hyperbaric oxygen therapy". Undersea and Hyperbaric Medicine. Undersea and Hyperbaric Medical Society, Inc. 30 (2): 147–53. PMID 12964858. Retrieved 20 October 2008.
- ^ Yildiz, S; Aktas, S; Cimsit, M; Ay, H; Togrol, E (2004). "Seizure incidence in 80,000 patient treatments with hyperbaric oxygen". Aviation, Space, and Environmental Medicine. 75 (11): 992–94. PMID 15559001. Retrieved 1 July 2009.
- ^ Bert, Paul (1943) [First published in French in 1878]. Barometric pressure: Researches in Experimental Physiology. Columbus, OH: College Book Company. Translated by: Hitchcock, Mary Alice; Hitchcock, Fred A
- ^ British Sub-aqua Club (1985). Sport diving : the British Sub-Aqua Club diving manual. London: Stanley Paul. p. 110. ISBN 0-09-163831-3. OCLC 12807848.
- ^ Behnke, Alfred R; Johnson, FS; Poppen, JR; Motley, EP (1935). "The effect of oxygen on man at pressures from 1 to 4 atmospheres". American Journal of Physiology. 110 (3): 565–72. doi:10.1152/ajplegacy.1934.110.3.565. Note: 1 atmosphere (atm) is 1.013 bars.
- ^ Behnke, Alfred R; Forbes, HS; Motley, EP (1935). "Circulatory and visual effects of oxygen at 3 atmospheres pressure". American Journal of Physiology. 114 (2): 436–42. doi:10.1152/ajplegacy.1935.114.2.436. Note: 1 atmosphere (atm) is 1.013 bars.
- ^ Donald 1992.
- ^ Taylor, Larry "Harris" (1993). "Oxygen Enriched Air: A New Breathing Mix?". IANTD Journal. Retrieved 29 May 2008.
- ^ Davis, Robert H (1955). Deep Diving and Submarine Operations (6th ed.). Tolworth, Surbiton, Surrey: Siebe Gorman & Company Ltd. p. 291.
- ^ Lambertsen, Christian J; Clark, John M; Gelfand, R (2000). "The Oxygen research program, University of Pennsylvania: Physiologic interactions of oxygen and carbon dioxide effects and relations to hyperoxic toxicity, therapy, and decompression. Summation: 1940 to 1999". EBSDC-IFEM Report No. 3-1-2000. Philadelphia, PA: Environmental Biomedical Stress Data Center, Institute for Environmental Medicine, University of Pennsylvania Medical Center.
- ^ Vann, Richard D (2004). "Lambertsen and O2: Beginnings of operational physiology". Undersea and Hyperbaric Medicine. 31 (1): 21–31. PMID 15233157. Retrieved 29 April 2008.
- ^ Clark & Lambertsen 1970.
- ^ Lang 2001, pp. 81–86.
- ^ Northway, WH; Rosan, RC; Porter, DY (1967). "Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia". New England Journal of Medicine. 276 (7): 357–68. doi:10.1056/NEJM196702162760701. PMID 5334613.
- ^ Shennan, AT; Dunn, MS; Ohlsson, A; Lennox, K; Hoskins, EM (1988). "Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period". Pediatrics. 82 (4): 527–32. doi:10.1542/peds.82.4.527. PMID 3174313. S2CID 2398582.
- ^ Palta, Mari; Sadek, Mona; Barnet, Jodi H; et al. (January 1998). "Evaluation of criteria for chronic lung disease in surviving very low birth weight infants. Newborn Lung Project". Journal of Pediatrics. 132 (1): 57–63. doi:10.1016/S0022-3476(98)70485-8. PMID 9470001.
- ^ Natoli, MJ; Vann, Richard D (1996). "Factors Affecting CNS Oxygen Toxicity in Humans". Report to the U.S. Office of Naval Research. Durham, NC: Duke University. Retrieved 29 April 2008.
- ^ Hof, DG; Dexter, JD; Mengel, CE (1971). "Effect of circadian rhythm on CNS oxygen toxicity". Aerospace Medicine. 42 (12): 1293–96. PMID 5130131.
- ^ Torley, LW; Weiss, HS (1975). "Effects of age and magnesium ions on oxygen toxicity in the neonate chicken". Undersea Biomedical Research. 2 (3): 223–27. PMID 15622741. Retrieved 20 September 2008.
- ^ Troy, SS; Ford, DH (1972). "Hormonal protection of rats breathing oxygen at high pressure". Acta Neurologica Scandinavica. 48 (2): 231–42. doi:10.1111/j.1600-0404.1972.tb07544.x. PMID 5061633. S2CID 28618515.
- ^ Hart, George B; Strauss, Michael B (2007). "Gender differences in human skeletal muscle and subcutaneous tissue gases under ambient and hyperbaric oxygen conditions". Undersea and Hyperbaric Medicine. 34 (3): 147–61. PMID 17672171. Retrieved 20 September 2008.
- ^ Shykoff, Barbara E (2007). "Performance of various models in predicting vital capacity changes caused by breathing high oxygen partial pressures". Nedu-Tr-07-13. Panama City, FL: U.S. Naval Experimental Diving Unit Technical Report. Retrieved 6 June 2008.
- ^ British Sub-Aqua Club (2006). "The Ocean Diver Nitrox Workshop" (PDF). British Sub-Aqua Club. p. 6. Archived from the original (PDF) on 16 July 2011. Retrieved 15 September 2010.
- ^ a b Bren, Linda (November–December 2002). "Oxygen Bars: Is a Breath of Fresh Air Worth It?". FDA Consumer. Vol. 36, no. 6. pp. 9–11. PMID 12523293. Retrieved 25 March 2020.
- ^ "O2 Planet – Exercise and Fitness Equipment". O2Planet LLC. 2006. Archived from the original on 15 April 2006. Retrieved 21 October 2008.
- ^ Verne, Jules (2004) [1872]. A Fantasy of Dr Ox. Hesperus Press. ISBN 978-1-84391-067-1. Retrieved 8 May 2009. Translated from French
- ^ Verne, Jules (1877) [1870]. "VIII" [At seventy-eight thousand one hundred and fourteen leagues]. Autour de la Lune [Round the Moon]. London: Ward Lock. ISBN 2-253-00587-8. Retrieved 2 September 2009. Translated from French
Sources[]
- Clark, James M; Thom, Stephen R (2003). "Oxygen under pressure". In Brubakk, Alf O; Neuman, Tom S (eds.). Bennett and Elliott's physiology and medicine of diving (5th ed.). United States: Saunders. pp. 358–418. ISBN 978-0-7020-2571-6. OCLC 51607923.
- Clark, John M; Lambertsen, Christian J (1970). "Pulmonary oxygen tolerance in man and derivation of pulmonary oxygen tolerance curves". IFEM Report No. 1-70. Philadelphia, PA: Environmental Biomedical Stress Data Center, Institute for Environmental Medicine, University of Pennsylvania Medical Center. Archived from the original on 7 October 2008. Retrieved 29 April 2008.
- Donald, Kenneth W (1947). "Oxygen Poisoning in Man: Part I". British Medical Journal. 1 (4506): 667–72. doi:10.1136/bmj.1.4506.667. PMC 2053251. PMID 20248086.
- Donald, Kenneth W (1947). "Oxygen Poisoning in Man: Part II". British Medical Journal. 1 (4507): 712–17. doi:10.1136/bmj.1.4507.712. PMC 2053400. PMID 20248096.
- Revised version of Donald's articles also available as:
- Donald, Kenneth W (1992). Oxygen and the diver. UK: Harley Swan, 237 pages. ISBN 1-85421-176-5. OCLC 26894235.
- Hamilton, Robert W; Thalmann, Edward D (2003). "Decompression practice". In Brubakk, Alf O; Neuman, Tom S (eds.). Bennett and Elliott's physiology and medicine of diving (5th ed.). United States: Saunders. pp. 475–79. ISBN 978-0-7020-2571-6. OCLC 51607923.
- Lang, Michael A, ed. (2001). DAN nitrox workshop proceedings. Durham, NC: Divers Alert Network, 197 pages. Archived from the original on 16 September 2011. Retrieved 20 September 2008.
- Regillo, Carl D; Brown, Gary C; Flynn, Harry W (1998). Vitreoretinal Disease: The Essentials. New York: Thieme, 693 pages. ISBN 978-0-86577-761-3. OCLC 39170393.
- U.S. Navy Supervisor of Diving (2011). U.S. Navy Diving Manual (PDF). SS521-AG-PRO-010 0910-LP-106-0957, revision 6 with Change A entered. U.S. Naval Sea Systems Command. Archived from the original (PDF) on 10 December 2014. Retrieved 29 January 2015.
{{cite book}}:|author=has generic name (help)
Further reading[]
- Lamb, John S. (1999). The Practice of Oxygen Measurement for Divers. Flagstaff: Best Publishing, 120 pages. ISBN 0-941332-68-3. OCLC 44018369.
- Lippmann, John; Bugg, Stan (1993). The Diving Emergency Handbook. Teddington, UK: Underwater World Publications. ISBN 0-946020-18-3. OCLC 52056845.
- Lippmann, John; Mitchell, Simon (2005). "Oxygen". Deeper into Diving (2nd ed.). Victoria, Australia: J.L. Publications. pp. 121–24. ISBN 0-9752290-1-X. OCLC 66524750.
External links[]
General
The following external site is a compendium of resources:
- Rubicon Research Repository – Online collection of the oxygen toxicity research
Specialised
The following external sites contain resources specific to particular topics:
- 2008 Divers Alert Network Technical Diving Conference – Video of "Oxygen Toxicity" lecture by Dr. Richard Vann (free download, mp4, 86MB).
- Nosek, Thomas M. "Section 4/4ch7/s4ch7_7". Essentials of Human Physiology. Archived from the original on 24 March 2016. – Discussion of the effects of breathing oxygen on the respiratory system.
- Rajiah, Prabhakar (11 March 2009). "Bronchopulmonary Dysplasia". eMedicine. WebMD. Retrieved 29 June 2009. – Clinical overview with references.
- Diving medicine
- Element toxicology
- Intensive care medicine
- Oxygen
- Respiratory diseases
- Neurobiological brain disorder