Perfluorotributylamine
| |
| Names | |
|---|---|
| Preferred IUPAC name
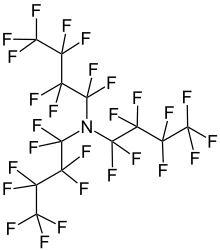
1,1,2,2,3,3,4,4,4-Nonafluoro-N,N-bis(nonafluorobutyl)butan-1-amine | |
| Other names
Fluorinert
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | PFTBA |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.659 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12F27N | |
| Molar mass | 671.096 g·mol−1 |
| Density | 1.884 g/mL |
| Melting point | −50 °C (−58 °F; 223 K) |
| Boiling point | 178 °C (352 °F; 451 K) |
| Insoluble | |
| Solubility in methanol and isopropyl alcohol | Insoluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Perfluorotributylamine (PFTBA), also referred to as FC43, is a colorless liquid with the formula N(C4F9)3. The compound consists of three butyl groups connected to an amine center, in which all of the hydrogen atoms have been replaced with fluorine. The compound is produced for the electronics industry, along with other perfluoroalkylamines. The high degree of fluorination significantly reduces the basicity of the central amine due to electron-withdrawing effects.[1]
Preparation[]
It is prepared by electrofluorination of tributylamine using hydrogen fluoride as solvent and source of fluorine:[2]
- N(C4H9)3 + 27 HF → N(C4F9)3 + 27 H2
Uses[]
The compound has two commercial uses. It is used as an ingredient in Fluosol, artificial blood. This application exploits the high solubility of oxygen and carbon dioxide in the solvent, as well as the low viscosity and toxicity.[3] It is also a component of Fluorinert coolant liquids. CPUs of some computers are immersed in this liquid to facilitate cooling.[2]
Niche[]
The compound is used as a calibrant[4] in gas chromatography when the analytical technique uses mass spectrometry as a detector to identify and quantify chemical compounds in gases or liquids. When undergoing ionization in the mass spectrometer, the compound decomposes in a repeatable pattern to form fragments of specific masses, which can be used to tune the mass response and accuracy of the mass spectrometer. Most commonly used ions are those with approximate mass of 69, 131, 219, 414 and 502 atomic mass units.
Safety[]
Fluorofluids are generally of very low toxicity, so much that they have been evaluated as synthetic blood.[2]
Environmental impact[]
It is a greenhouse gas with warming properties more than 7,000 times that of carbon dioxide over a 100-year period,[5][6] and, as such, is the most potent greenhouse gas ever discovered.[7] Its concentration in the atmosphere is approximately 0.18 parts per trillion. The compound can persist in the atmosphere for up to 500 years. Sulfur hexafluoride, however, has a GWP of 23,900,[8] which would make it much more powerful.

Global warming potential of greenhouse gases and PFTBA
See also[]
References[]
- ^ "Tuning basicity | Cambridge MedChem Consulting". www.cambridgemedchemconsulting.com. Retrieved 2020-08-11.
- ^ a b c Michael G. Costello, Richard M. Flynn, John G. Owens (2001). "Fluoroethers and Fluoroamines". Kirk-Othmer Encyclopedia of Chemical Technology. Weinstein: Wiley-VCH. doi:10.1002/0471238961.0612211506122514.a01.pub2. ISBN 978-0471238966.CS1 maint: uses authors parameter (link)
- ^ Garrelts, J. C. (1990). "Fluosol: An oxygen-delivery fluid for use in percutaneous transluminal coronary angioplasty". DICP: The Annals of Pharmacotherapy. 24 (11): 1105–1112. doi:10.1177/106002809002401116. PMID 2275237. S2CID 38969204.
- ^ Dunnivant, Frank and Ginsbach, Jake. "GAS CHROMATOGRAPHY, LIQUID CHROMATOGRAPHY, CAPILLARY Electrophoresis - MASS SPECTROMETRY A BASIC INTRODUCTION", Chapter 7, ISBN 978-0-9882761-0-9, [1]., Nov. 2012.
- ^ Hong, A. C.; Young, C. J.; Hurley, M. D.; Wallington, T. J.; Mabury, S. A. (2013). "Perfluorotributylamine: A novel long-lived greenhouse gas". Geophysical Research Letters. 40 (22): 6010–6015. Bibcode:2013GeoRL..40.6010H. doi:10.1002/2013GL058010.
- ^ Goldenberg, Suzanne (10 December 2013). "Newly discovered greenhouse gas '7,000 times more powerful than CO2'". The Guardian. Retrieved 11 December 2013.
- ^ Goldenberg, Suzanne (11 December 2013). "Newly Discovered Greenhouse Gas "7,000 Times More Powerful than CO2"". Mother Jones. Retrieved 12 December 2013.
- ^ "2.10.2 Direct Global Warming Potentials". Intergovernmental Panel on Climate Change. 2007. Retrieved 22 February 2013.
- Greenhouse gases
- Perfluorinated compounds
- Amines
- Coolants
