Pyridinium chlorochromate

| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Pyridinium chlorochromate
| |||
| Other names
PCC; Corey-Suggs reagent
| |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.043.253 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| C5H6ClCrNO3 | |||
| Molar mass | 215.56 g/mol | ||
| Appearance | yellow-orange solid[1] | ||
| Melting point | 205 °C (401 °F; 478 K) | ||
| Solubility in other solvents | soluble in acetone, acetonitrile, THF | ||
| Hazards | |||
| Main hazards | Suspected carcinogen and environmental pollutant | ||
| Safety data sheet | external SDS | ||
| GHS pictograms |    
| ||
| GHS Signal word | Danger | ||
GHS hazard statements
|
H350, H272, H317, H410 | ||
| P201, P280, P273, P221, P308+313, P302+352 | |||
| NFPA 704 (fire diamond) | 
2
1
2 OX | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula [C5H5NH]+[CrO3Cl]−. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective.[1]
Structure and preparation[]
PCC consists of a pyridinium cation, [C5H5NH]+, and a tetrahedral chlorochromate anion, [CrO3Cl]−. Related salts are also known, such as 1-butylpyridinium chlorochromate, [C5H5N(C4H9)][CrO3Cl] and potassium chlorochromate.
PCC is commercially available. Discovered by accident,[3] the reagent was originally prepared via addition of pyridine into a cold solution of chromium trioxide in concentrated hydrochloric acid:[4]
- C5H5N + HCl + CrO3 → [C5H5NH][CrO3Cl]
In one alternative method, formation of chromyl chloride (CrO2Cl2) fume during the making of the aforementioned solution was minimized by simply changing the order of addition: a cold solution of pyridine in concentrated hydrochloric acid was added to solid chromium trioxide under stirring.[5]
Uses[]
Oxidation of alcohols[]
PCC is used as an oxidant. In particular, it has proven to be highly effective in oxidizing primary and secondary alcohols to aldehydes and ketones, respectively. The reagent is more selective than the related Jones reagent, so there is little chance of over-oxidation to form carboxylic acids as long as water is not present in the reaction mixture. A typical PCC oxidation involves addition of an alcohol to a suspension of PCC in dichloromethane.[6][7][8] The general reaction is:
- 2 [C5H5NH][CrO3Cl] + 3 R2CHOH → 2 [C5H5NH]Cl + Cr2O3 + 3 R2C=O + 3 H2O
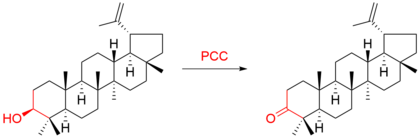
For example, the triterpene lupeol was oxidized to :[9]
Babler oxidation[]
With tertiary alcohols, the chromate ester formed from PCC can isomerize via a [3,3]-sigmatropic reaction and following oxidation yield an enone, in a reaction known as the Babler oxidation:

This type of oxidative transposition reaction has been synthetically utilized, e.g. for the synthesis of morphine.[10]
Using other common oxidants in the place of PCC usually leads to dehydration, because such alcohols cannot be oxidized directly.
Other reactions[]
PCC also converts suitable unsaturated alcohols and aldehydes to cyclohexenones. This pathway, an oxidative cationic cyclization, is illustrated by the conversion of (−)-citronellol to (−)-pulegone.
PCC also effects allylic oxidations, for example, in conversion of dihydrofurans to furanones.[1]
Related reagents[]
Other more convenient or less toxic reagents for oxidizing alcohols include dimethyl sulfoxide, which is used in Swern and Pfitzner–Moffatt oxidations, and hypervalent iodine compounds, such as the Dess–Martin periodinane.
Safety[]
One disadvantage to the use of PCC is its toxicity, which it shares with other hexavalent chromium compounds.
See also[]
- Oxidation with chromium(VI)-amine complexes
- Babler oxidation
References[]
- ^ Jump up to: a b c Piancatelli, G.; Luzzio, F. A. (2007). "Pyridinium Chlorochromate". e-EROS Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/9780470842898.rp288.pub2. ISBN 978-0471936237.
- ^ "Safety Data Sheet". Acros Organics. 2015. Retrieved 2016-06-10.
- ^ Lowe, Derek. "The Old Stuff". In The Pipeline. Science. Retrieved 2015-11-21.
- ^ Corey, E. J.; Suggs, J. W. (1975). "Pyridinium Chlorochromate. An Efficient Reagent for Oxidation of Primary and Secondary Alcohols to Carbonyl Compounds". Tetrahedron Lett. 16 (31): 2647–2650. doi:10.1016/S0040-4039(00)75204-X.
- ^ Agarwal, S.; Tiwari, H. P.; Sharma, J. P. (1990). "Pyridinium Chlorochromate: An Improved Method for Its Synthesis and Use of Anhydrous Acetic Acid as Catalyst for Oxidation Reactions". Tetrahedron. 46 (12): 4417–4420. doi:10.1016/S0040-4020(01)86776-4.
- ^ Paquette, L. A.; Earle, M. J.; Smith, G. F. (1996). "(4R)-(+)-tert-Butyldimethylsiloxy-2-cyclopenten-1-one". Organic Syntheses. 73: 36.CS1 maint: multiple names: authors list (link); Collective Volume, 9, p. 132
- ^ Tu, Y.; Frohn, M.; Wang, Z.-X.; Shi, Y. (2003). "Synthesis of 1,2:4,5-Di-O-isopropylidene-D-erythro-2,3-hexodiulo-2,6-pyranose. A Highly Enantioselective Ketone Catalyst for Epoxidation". Organic Syntheses. 80: 1.CS1 maint: multiple names: authors list (link)
- ^ White, J. D.; Grether, U. M.; Lee, C.-S. (2005). "(R)-(+)-3,4-Dimethylcyclohex-2-en-1-one". Organic Syntheses. 82: 108.CS1 maint: multiple names: authors list (link); Collective Volume, 11, p. 100
- ^ Lao, A.; Fujimoto, Y.; Tatsuno, T. (1984). "Studies on the Constituents of Artemisia argyi Lévl & Vant". Chem. Pharm. Bull. 32 (2): 723–727. doi:10.1248/cpb.32.723. Retrieved 2016-06-05.
- ^ Killoran, Patrick M.; Rossington, Steven B.; Wilkinson, James A.; Hadfield, John A. (2016). "Expanding the scope of the Babler–Dauben oxidation: 1,3-oxidative transposition of secondary allylic alcohols". Tetrahedron Letters. 57 (35): 3954–3957. doi:10.1016/j.tetlet.2016.07.076.
Further reading[]
- Tojo, G.; Fernández, M. (2006). Tojo, G. (ed.). Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice. Basic Reactions in Organic Synthesis. New York: Springer. ISBN 978-0-387-23607-0.
External links[]
- Chromates
- Oxidizing agents
- IARC Group 1 carcinogens
- Pyridinium compounds