Chromium(III) acetate
| |

| |
| Names | |
|---|---|
| IUPAC name
Chromium(III) acetate hydrate
| |
| Other names
chromic acetate,
chromium triacetate, chromium(III) ethanoate | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.012.646 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H36ClCr3O22 | |
| Molar mass | 723.84 g·mol−1 |
| Appearance | grayish-green to blueish-green solid |
| Density | 1.662 g/cm3 |
| Melting point | 1,152[1] °C (2,106 °F; 1,425 K) |
| -5104.0·10−6 cm3/mol | |
| Structure | |
| octahedral | |
| Related compounds | |
Related compounds
|
Manganese(III) acetate Iron(III) acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
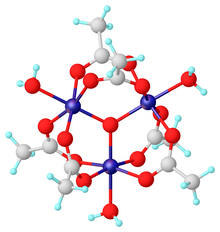
Chromium(III) acetate, commonly known as basic chromium acetate,[2] describes a family of salts where the cation has the formula [Cr3O(O2CCH3)6(OH2)3]+. The trichromium cation is encountered with a variety of anions, such as chloride and nitrate. Data in the table above are for the chloride hexahydrate, [Cr3O(O2CCH3)6(OH2)3]Cl(H2O)6.
Salts of basic chromium acetate has long attracted interest because of its distinctive structure, which features octahedral Cr(III) centers, a triply bridging oxo ligand, six acetate ligands, and three aquo ligands.[2] The same structure is shared with basic iron acetate and basic manganese acetate.[2][3] Little evidence exists for a simple chromium(III) acetate, i.e. lacking the oxo ligand.[4] Chromium(III) acetate is a blue/grey-green powder, which is soluble in water. It is still[3] prepared according to the original procedure from 1909.[5]

See also[]
References[]
- ^ "Chromium (III) compounds". National Pollutant Inventory. Australian Government Department of Agriculture, Water and the Environment. Retrieved 14 April 2021.
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b Kurt J. Schenk, Hans U. Güdel (1982). "Low-temperature structural and spectroscopic properties of [Cr3O(CH3COO)6(H2O)3]Cl.6H2O". Inorg. Chem. 21 (6): 2253–2256. doi:10.1021/ic00136a025.CS1 maint: uses authors parameter (link)
- ^ Erre, Liliana Strinna; Micera, Giovanni; Glowiak, Tadeusz; Kozlowski, Henry (April 1997). "Chromium (III) Acetate, Chromium (III) Acetate Hydroxide, or µ3-Oxo-esakis-(µ2-acetato-O,O') - triaqua-trichromium (III) Acetate? Determining the Structure of a Complex Compound by Analytical and Spectroscopic Methods". Journal of Chemical Education. 74 (4): 432. doi:10.1021/ed074p432.
- ^ R. Weinland P. Dinkelacker (1909). "Über Salze einer Hexaacetato(formiato)‐trichrombase. II". Berichte der Deutschen Chemischen Gesellschaft. 42 (3): 2997–3018. doi:10.1002/cber.19090420318.CS1 maint: uses authors parameter (link)
- Chromium(III) compounds
- Acetates
- Chromium–oxygen compounds