Chromium hexacarbonyl
| |
| Names | |
|---|---|
| IUPAC name
Hexacarbonylchromium
| |
| Other names
Chromium carbonyl
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.032.579 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cr(CO)6 | |
| Molar mass | 220.057 g/mol |
| Appearance | colorless crystals |
| Density | 1.77 g/cm3, solid |
| Melting point | 90 °C (194 °F; 363 K) |
| Boiling point | 210 °C (410 °F; 483 K) (decomposes) |
| insoluble | |
| Solubility | soluble in organic solvents |
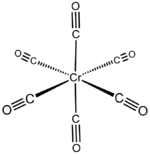
| Structure | |
| orthrhombic | |
| octahedral | |
Dipole moment
|
0 D |
| Hazards | |
| Main hazards | Toxic |
| Safety data sheet (SDS) | Oxford MSDS |
| NFPA 704 (fire diamond) | 
2
1
0 |
| Flash point | 210 °C (410 °F; 483 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
150 mg/kg (oral, mouse) 230 mg/kg (oral, rat) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3[1] |
REL (Recommended)
|
TWA 0.5 mg/m3[1] |
IDLH (Immediate danger)
|
250 mg/m3[1] |
| Related compounds | |
Other cations
|
Molybdenum hexacarbonyl Tungsten hexacarbonyl |
Related compounds
|
Vanadium hexacarbonyl Dimanganese decacarbonyl |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chromium carbonyl, also known as chromium hexacarbonyl, is the chemical compound with the formula Cr(CO)6. At room temperature the solid is stable to air, although it does have a high vapor pressure and sublimes readily. Cr(CO)6 is zerovalent, meaning that Cr has an oxidation state of zero, and it is a homoleptic complex, which means that all the ligands are identical. The complex is octahedral with Cr–C and C–O distances of 1.91 and 1.14 Å, respectively.[2]
History[]
The synthesis of Cr(CO)6 was described in a series of papers published in 1926–7. The procedure involves treatment of Cr(III) salts with high pressures of carbon monoxide using phenyl magnesium bromides as a reductant.[3][4]
Reactions[]
Pentacarbonyl derivatives[]
When heated or UV-irradiated in tetrahydrofuran (THF) solution, Cr(CO)6 converts to Cr(CO)5(THF) with loss of one CO ligand. The THF ligand is readily displaced. Often the THF complex is generated and used in situ.[5]
UV-irradiation of frozen solutions of chromium hexacarbonyl affords a variety of labile adducts, including labile but complexes with some noble gases.[6]
Arene derivatives[]
Heating a solution of Cr(CO)6 in an aromatic solvent results in replacement of three CO ligands. The reactions are especially favorable for electron-rich arenes:
- Cr(CO)6 + C6H5R → Cr(CO)3(C6H5R) + 3 CO
The products are "piano-stool" complexes. These species are typically yellow solids. One example is (benzene)chromium tricarbonyl.
Fischer carbenes and carbynes[]
Alkyl and aryl organolithium reagents (RLi) add to Cr(CO)6 to give anionic acyl complexes.[7] These anionic species in turn react with alkylating agents such as Me3O+ to form (OC)5Cr=C(OMe)R to give Fischer carbene complexes:[8]
Cyclopentadienyl derivatives[]
Treatment of chromium hexacarbonyl with sodium cyclopentadienide gives NaCr(CO)3(C5H5). Oxidation of this salt affords cyclopentadienylchromium tricarbonyl dimer (Cp2Cr2(CO)6). This complex is distinctive because it exists in measureable equilibrium with the monometallic Cr(I) radical CpCr(CO)3.[9]
Safety[]
In common with many of the other homoleptic metal carbonyls (e.g. nickel carbonyl and iron carbonyl), chromium hexacarbonyl is toxic and thought to be carcinogenic. Its vapor pressure is relatively high for a metal complex, 1 mmHg (130 Pa) at 36 °C).[10]
References[]
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0141". National Institute for Occupational Safety and Health (NIOSH).
- ^ Whitaker, A.; Jeffery, J. W. (1967). "The Crystal Structure of Chromium Hexacarbonyl". Acta Crystallogr. 23 (6): 977–984. doi:10.1107/S0365110X67004153.
- ^ Job, Andre; Cassal, Antoine. (1927). "Chromium Carbonyl". Bulletin de la Société Chimique de France. 41: 1041.
- ^ Owen, B. B.; English, J.; Cassidy, H. G.; Dundon, C. V. (1950). "Chromium Hexacarbonyl". Inorganic Syntheses. Inorganic Syntheses. 3. pp. 156–160. doi:10.1002/9780470132340.ch42. ISBN 9780470132340.
- ^ Costamagna, J. A.; Granifo, J. (1985). "(Substituted Thiourea)Pentacarbonylchromium(0) Complexes". Inorganic Syntheses. Inorganic Syntheses. 23. pp. 1–4. doi:10.1002/9780470132548.ch1. ISBN 9780470132548.
- ^ Perutz, Robin N.; Turner, James J. (1975). "Photochemistry of the Group 6 Hexacarbonyls in Low-Temperature Matrixes. III. Interaction of the Pentacarbonyls with Noble Gases and Other Matrixes". Journal of the American Chemical Society. 97 (17): 4791–800. doi:10.1021/ja00850a001.CS1 maint: uses authors parameter (link)
- ^ Elschenbroich, C. (2006). Organometallics. Weinheim: Wiley-VCH. ISBN 978-3-527-29390-2.
- ^ Herndon, James W. (2001). "Pentacarbonyl(methoxyphenylcarbene)chromium(0)". e-EROS Encyclopedia of Reagents for Organic Synthesis.
- ^ Manning, A. R.; Hacket, Paul; Birdwhistell, Ralph (1990). "Hexacarbonylbis(η5‐Cyclopentadienyl)Dichromium, Molybdenum, and Tungsten and their Analogs, M2(η5‐C5H4R)2(CO)6 (M = Cr, Mo, and W; R = H, Me or PhCH2)". Inorganic Syntheses. 28: 148–149. doi:10.1002/9780470132593.ch39. ISBN 9780470132593.
- ^ Patnaik, Pradyot (2003). "Chromium hexacarbonyl". Handbook of Inorganic Chemicals. McGraw-Hill Professional. pp. 222–223. ISBN 978-0-07-049439-8.
External links[]
- Carbonyl complexes
- Chromium complexes
- Octahedral compounds
- Organochromium compounds

