Retrosynthetic analysis
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. Each precursor material is examined using the same method. This procedure is repeated until simple or commercially available structures are reached. These simpler/commercially available compounds can be used to form a synthesis of the target molecule. E.J. Corey formalized this concept in his book The Logic of Chemical Synthesis.[1][2][3]
The power of retrosynthetic analysis becomes evident in the design of a synthesis. The goal of retrosynthetic analysis is a structural simplification. Often, a synthesis will have more than one possible synthetic route. Retrosynthesis is well suited for discovering different synthetic routes and comparing them in a logical and straightforward fashion.[4] A database may be consulted at each stage of the analysis, to determine whether a component already exists in the literature. In that case, no further exploration of that compound would be required. If that compound exists, it can be a jumping point for further steps developed to reach a synthesis.
Definitions[]
- Disconnection
- A retrosynthetic step involving the breaking of a bond to form two (or more) synthons.
- Retron
- A minimal molecular substructure that enables certain transformations.
- Retrosynthetic tree
- A directed acyclic graph of several (or all) possible retrosyntheses of a single target.
- Synthon
- A fragment of a compound that assists in the formation of a synthesis, derived from that target molecule. A synthon and the corresponding commercially available synthetic equivalent are shown below:
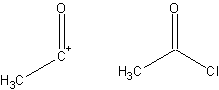
- Target
- The desired final compound.
- Transform
- The reverse of a synthetic reaction; the formation of starting materials from a single product.
Example[]
An example will allow the concept of retrosynthetic analysis to be easily understood.

In planning the synthesis of phenylacetic acid, two synthons are identified. A nucleophilic "-COOH" group, and an electrophilic "PhCH2+" group. Of course, both synthons do not exist per se; synthetic equivalents corresponding to the synthons are reacted to produce the desired product. In this case, the cyanide anion is the synthetic equivalent for the −COOH synthon, while benzyl bromide is the synthetic equivalent for the benzyl synthon.
The synthesis of phenylacetic acid determined by retrosynthetic analysis is thus:
- PhCH2Br + NaCN → PhCH2CN + NaBr
- PhCH2CN + 2 H2O → PhCH2COOH + NH3

In fact, phenylacetic acid has been synthesized from benzyl cyanide,[5] itself prepared by the analogous reaction of benzyl chloride with sodium cyanide.[6]
Strategies[]
Functional group strategies[]
Manipulation of functional groups can lead to significant reductions in molecular complexity.
Stereochemical strategies[]
Numerous chemical targets have distinct stereochemical demands. Stereochemical transformations (such as the Claisen rearrangement and Mitsunobu reaction) can remove or transfer the desired chirality thus simplifying the target.
Structure-goal strategies[]
Directing a synthesis toward a desirable intermediate can greatly narrow the focus of analysis. This allows bidirectional search techniques.
Transform-based strategies[]
The application of transformations to retrosynthetic analysis can lead to powerful reductions in molecular complexity. Unfortunately, powerful transform-based retrons are rarely present in complex molecules, and additional synthetic steps are often needed to establish their presence.
Topological strategies[]
The identification of one or more key bond disconnections may lead to the identification of key substructures or difficult to identify rearrangement transformations in order to identify the key structures.
- Disconnections that preserve ring structures are encouraged.
- Disconnections that create rings larger than 7 members are discouraged.
- Disconnection involves creativity.
See also[]
References[]
- ^ E. J. Corey, X-M. Cheng (1995). The Logic of Chemical Synthesis. New York: Wiley. ISBN 978-0-471-11594-6.
- ^ E. J. Corey (1988). "Retrosynthetic Thinking – Essentials and Examples". Chem. Soc. Rev. 17: 111–133. doi:10.1039/CS9881700111.
- ^ E. J. Corey (1991). "The Logic of Chemical Synthesis: Multistep Synthesis of Complex Carbogenic Molecules (Nobel Lecture)" (Reprint). Angewandte Chemie International Edition in English. 30 (5): 455–465. doi:10.1002/anie.199104553.
- ^ James Law et.al:"Route Designer: A Retrosynthetic Analysis Tool Utilizing Automated Retrosynthetic Rule Generation", Journal of Chemical Information and Modelling (ACS JCIM) Publication Date (Web): February 6, 2009; doi:10.1021/ci800228y, http://pubs.acs.org/doi/abs/10.1021/ci800228y
- ^ Wilhelm Wenner (1963). "Phenylacetamide". Organic Syntheses.; Collective Volume, 4, p. 760
- ^ Roger Adams; A. F. Thal (1941). "Benzyl Cyanide". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 1, p. 107
External links[]
| Wikiquote has quotations related to: Retrosynthetic analysis |
- Centre for Molecular and Biomolecular Informatics
- Presentation on ARChem Route Designer, ACS, Philadelphia, September 2008 for more info on ARChem see the SimBioSys pages.
- Manifold, Software freely available for academic users developed by PostEra
- Retrosynthesis planning tool: ICSynth by InfoChem
- Spaya, Software freely available proposed by Iktos
- Chemical synthesis
- Organic chemistry
- Chemical reaction engineering