Sarecycline
 | |
| Clinical data | |
|---|---|
| Pronunciation | sar"e sye' kleen |
| Trade names | Seysara |
| Other names | P-005672 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618068 |
| License data |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.241.852 |
| Chemical and physical data | |
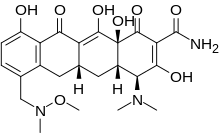
| Formula | C24H29N3O8 |
| Molar mass | 487.509 g·mol−1 |
| 3D model (JSmol) | |
| |
Sarecycline is a narrow-spectrum tetracycline-derived antibiotic.[1][2] It is specifically designed for the treatment of acne, and was approved by the FDA in October 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years of age and older.[3] Two randomized and well-controlled clinical trials reported efficacy data on both facial and truncal acne (back and chest).[4] Efficacy was assessed in a total of 2002 subjects 9 years of age and older.[3] Unlike other tetracycline-class antibiotics, sarecycline has a long C7 moiety that extends into and directly interact with the bacterial messenger RNA (mRNA).[5] The spectrum of activity is limited to clinically relevant Gram-positive bacteria, mainly Cutibacterium acnes, with little or no activity against Gram-negative bacterial microflora commonly found in the human gastrointestinal tract.[6] Sarecycline has not been tested in spirochetes.
References[]
- ^ Zhanel G, Critchley I, Lin LY, Alvandi N (January 2019). "Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris". Antimicrobial Agents and Chemotherapy. 63 (1). doi:10.1128/AAC.01297-18. PMC 6325184. PMID 30397052.
- ^ PubChem. "Sarecycline". pubchem.ncbi.nlm.nih.gov. Retrieved 2020-09-05.
- ^ a b "FDA-approved Labeling-Package Insert for Seysara" (PDF). Drugs@FDA. June 2020. Retrieved September 5, 2020.
- ^ Moore, Angela; Green, Lawrence J.; Bruce, Suzanne; Sadick, Neil; Tschen, Eduardo; Werschler, Philip; Cook-Bolden, Fran E.; Dhawan, Sunil S.; Forsha, Douglass; Gold, Michael H.; Guenthner, Scott (2018-09-01). "Once-Daily Oral Sarecycline 1.5 mg/kg/day Is Effective for Moderate to Severe Acne Vulgaris: Results from Two Identically Designed, Phase 3, Randomized, Double-Blind Clinical Trials". Journal of Drugs in Dermatology. 17 (9): 987–996. ISSN 1545-9616. PMID 30235387.
- ^ Batool, Zahra; Lomakin, Ivan B.; Polikanov, Yury S.; Bunick, Christopher G. (2020-08-25). "Sarecycline interferes with tRNA accommodation and tethers mRNA to the 70S ribosome". Proceedings of the National Academy of Sciences. 117 (34): 20530–20537. doi:10.1073/pnas.2008671117. ISSN 0027-8424. PMC 7456112. PMID 32817463.
- ^ Zhanel, George; Critchley, Ian; Lin, Lynn-Yao; Alvandi, Nancy (2019-01-01). "Microbiological Profile of Sarecycline, a Novel Targeted Spectrum Tetracycline for the Treatment of Acne Vulgaris". Antimicrobial Agents and Chemotherapy. 63 (1). doi:10.1128/AAC.01297-18. ISSN 0066-4804. PMC 6325184. PMID 30397052.
External links[]
- "Sarecycline". Drug Information Portal. U.S. National Library of Medicine.
- Tetracycline antibiotics
- Antiinfective agent stubs