Selenide
A selenide is a chemical compound containing a selenium anion with oxidation number of −2 (Se2−), much as sulfur does in a sulfide. The chemistry of the selenides and sulfides is similar. Similar to sulfide, in aqueous solution, the selenide ion, Se2−, is prevalent only in very basic conditions. In neutral conditions, hydrogen selenide ion, HSe−, is most common. In acid conditions, hydrogen selenide, H2Se, is formed.
Some selenides are reactive to oxidation by air. Owing to the greater reducing power of selenide, metal selenides are more easily decomposed to the elements than are sulfides (tellurides are even more labile). Selenides of electropositive metals: such as aluminium selenide readily hydrolyse, even in moist air, evolving toxic hydrogen selenide gas.
Pure selenide minerals are rare, instead selenium tends to partially substitute for sulfide in many sulfide minerals. The degree of substitution is only of commercial interest for copper sulfide ores, in which case selenium is recovered as a by-product of copper refining. Some selenide minerals include ferroselite and umangite.[1]
Reactions[]
Alkali metal selenides react with elemental selenium to give salts of polyselenide.
Metal selenide[]
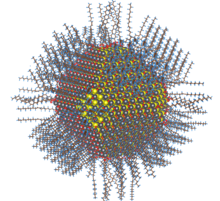
Metal selenide quantum dots and nanoparticles can be prepared by a variety of synthetic methods are available, many of which require high temperatures and hazardous precursor compounds.[2] The particles can be adapted for a variety of applications by varying the ligands coordinated to the positively-charged outer layer. Many ligand-exchange reactions are available for use, trading X, L, and Z-type ligands, the mechanism for which is still under study.[3]
Applications[]
Quantum dots based on metal selenides have been extensively for their distinctive spectral properties.[4] Core-shell alloys of cadmium sulfide and selenide are of interest in imaging and phototherapy.[5]
Examples[]
- Gallium(II) selenide
- Indium(III) selenide
- Sodium selenide
- Cadmium selenide
- Zinc selenide
- Lead selenide
- Copper selenide
References[]
- ^ Bernd E. Langner "Selenium and Selenium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a23_525.
- ^ Chen, Ou; Chen, Xian; Yang, Yongan; Lynch, Jared; Wu, Huimeng; Zhuang, Jiaqi; Cao, Y. Charles (2008). "Synthesis of Metal-Selenide Nanocrystals Using Selenium Dioxide as the Selenium Precursor". Angewandte Chemie International Edition. 47 (45): 8638–8641. doi:10.1002/anie.200804266. ISSN 1433-7851. PMID 18850601.
- ^ Anderson, Nicholas C.; Owen, Jonathan S. (2013-01-08). "Soluble, Chloride-Terminated CdSe Nanocrystals: Ligand Exchange Monitored by 1H and 31P NMR Spectroscopy". Chemistry of Materials. 25 (1): 69–76. doi:10.1021/cm303219a. ISSN 0897-4756.
- ^ Larson, Daniel R.; Zipfel, Warren R.; Williams, Rebecca M.; Clark, Stephen W.; Bruchez, Marcel P.; Wise, Frank W.; Webb, Watt W. (2003-05-30). "Water-Soluble Quantum Dots for Multiphoton Fluorescence Imaging in Vivo". Science. 300 (5624): 1434–1436. Bibcode:2003Sci...300.1434L. doi:10.1126/science.1083780. ISSN 0036-8075. PMID 12775841.
- ^ Hessel, Colin M.; Pattani, Varun P.; Rasch, Michael; Panthani, Matthew G.; Koo, Bonil; Tunnell, James W.; Korgel, Brian A. (2011-06-08). "Copper Selenide Nanocrystals for Photothermal Therapy". Nano Letters. 11 (6): 2560–2566. Bibcode:2011NanoL..11.2560H. doi:10.1021/nl201400z. ISSN 1530-6984. PMC 3111000. PMID 21553924.
External links[]
- Selenides
- Chalcogenides
- Solar cells
- Selenium(−II) compounds