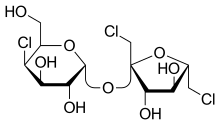
Sucralose
| |

| |
| Names | |
|---|---|
| IUPAC name
1,6-Dichloro-1,6-dideoxy-β-D-fructofuranosyl-4-chloro-4-deoxy-α-D-galactopyranoside
| |
| Preferred IUPAC name
(2R,3R,4R,5R,6R)-2-{[(2R,3S,4S,5S)-2,5-Bis(chloromethyl)-3,4-dihydroxyoxolan-2-yl]oxy}-5-chloro-6-(hydroxymethyl)oxane-3,4-diol | |
| Other names
1′,4,6′-Trichlorogalactosucrose; Trichlorosucrose; E955; 4,1′,6′-Trichloro-4,1′,6′-trideoxygalactosucrose; TGS; Splenda[2]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.054.484 |
| EC Number |
|
| E number | E955 (glazing agents, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H19Cl3O8 | |
| Molar mass | 397.64 g/mol |
| Appearance | Off-white to white powder |
| Odor | Odorless |
| Density | 1.69 g/cm3 |
| Melting point | 125 °C (257 °F; 398 K) |
| 283 g/L (20°C) | |
| Acidity (pKa) | 12.52±0.70 |
| Hazards | |
| NFPA 704 (fire diamond) | 
1
1
0 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sucralose is an artificial sweetener and sugar substitute. The majority of ingested sucralose is not broken down by the body, so it is noncaloric.[3] In the European Union, it is also known under the E number E955. It is produced by chlorination of sucrose, selectively replacing three of the hydroxy groups in the C1, C4, and C6 positions to give a 1,6-dichloro-1,6-dideoxyfructose–4-chloro-4-deoxygalactose disaccharide. Sucralose is about 320 to 1,000 times sweeter than sucrose,[4] three times as sweet as both aspartame and acesulfame potassium, and twice as sweet as sodium saccharin. Evidence of benefit is lacking for long-term weight loss with some data supporting weight gain and heart disease risks.[5]
While sucralose is largely considered shelf-stable and safe for use at elevated temperatures (such as in baked goods), there is some evidence that it begins to break down at temperatures above 119 degrees Celsius.[6][7] The commercial success of sucralose-based products stems from its favorable comparison to other low-calorie sweeteners in terms of taste, stability, and safety.[8] It is commonly sold under the Splenda brand name. Canderel Yellow also contains sucralose, but the original Canderel and Green Canderel do not.
Uses[]
Sucralose is used in many food and beverage products because it is a no-calorie sweetener, does not promote dental cavities,[9] is safe for consumption by diabetics and nondiabetics,[10][11] and does not affect insulin levels,[12] although the powdered form of sucralose-based sweetener product Splenda (as most other powdered sucralose products) contains 95% (by volume) bulking agents dextrose and maltodextrin that do affect insulin levels. Sucralose is used as a replacement for, or in combination with, other artificial or natural sweeteners such as aspartame, acesulfame potassium or high-fructose corn syrup. Sucralose is used in products such as candy, breakfast bars, coffee pods, and soft drinks. It is also used in canned fruits wherein water and sucralose take the place of much higher calorie corn syrup-based additives. Sucralose mixed with maltodextrin or dextrose (both made from corn) as bulking agents is sold internationally by McNeil Nutritionals under the Splenda brand name. In the United States and Canada, this blend is increasingly found in restaurants, in yellow packets, in contrast to the blue packets commonly used by aspartame and the pink packets used by those containing saccharin sweeteners; in Canada, yellow packets are also associated with the SugarTwin brand of cyclamate sweetener.
Cooking[]
Sucralose is available in a granulated form that allows for same-volume substitution with sugar. This mix of granulated sucralose includes fillers, all of which rapidly dissolve in water. While the granulated sucralose provides apparent volume-for-volume sweetness, the texture in baked products may be noticeably different. Sucralose is not hygroscopic, which can lead to baked goods that are noticeably drier and manifest a less dense texture than those made with sucrose. Unlike sucrose, which melts when baked at high temperatures, sucralose maintains its granular structure when subjected to dry, high heat (e.g., in a 350 °F or 180 °C oven). Furthermore, in its pure state, sucralose begins to decompose at 119 °C or 246 °F.[6] Thus, in some recipes, such as crème brûlée, which require sugar sprinkled on top to partially or fully melt and crystallize, substituting sucralose will not result in the same surface texture, crispness, or crystalline structure.
Safety evaluation[]
Sucralose has been accepted as safe by several food safety regulatory bodies worldwide, including the FDA, the Joint FAO/WHO Expert Committee Report on Food Additives, the European Union's Scientific Committee on Food, Health Protection Branch of Health and Welfare Canada, and Food Standards Australia New Zealand. According to the Canadian Diabetes Association, the amount of sucralose that can be consumed over a person's lifetime without any adverse effects is 900 mg per kg of body weight per day.[13][14]
"In determining the safety of sucralose, the FDA reviewed data from more than 110 studies in humans and animals. Many of the studies were designed to identify possible toxic effects, including carcinogenic, reproductive, and neurological effects. No such effects were found, and FDA's approval is based on the finding that sucralose is safe for human consumption." For example, McNeil Nutritional LLC studies – submitted as part of its U.S. FDA Food Additive Petition 7A3987 – indicated that "in the 2-year rodent bioassays ... there was no evidence of carcinogenic activity for either sucralose or its hydrolysis products ..."[15][16]
The FDA approval process indicated that consuming sucralose in typical amounts as a sweetener was safe.[14][15] When the estimated daily intake is compared to the intake at which adverse effects are seen (known as the "highest no-effects limit", or HNEL at 1500 mg/kg BW/day,[15] a large margin of safety exists. The bulk of sucralose ingested is not absorbed by the gastrointestinal tract (gut) and is directly excreted in the feces, while 11–27% of it is absorbed.[14][15] The amount absorbed from the gut is largely removed from the blood by the kidneys and eliminated in the urine, with 20–30% of the absorbed sucralose being metabolized.[14][15]
Research revealed that when sucralose is heated to above 248 °F (120 °C), it may dechlorinate and decompose into compounds that could be harmful enough to risk consumer health.[14][15] The risk and intensity of this adverse effect is suspected to increase with rising temperatures.[17] The German Federal Institute for Risk Assessment published an advisory warning that cooking with sucralose could possibly lead to the creation of potentially carcinogenic chloropropanols, polychlorinated dibenzodioxins and polychlorinated dibenzofurans, recommending that manufacturers and consumers avoid baking, roasting, or deep frying any sucralose-containing foods until a more conclusive safety report is available.[18] Furthermore, adding sucralose to food that has not cooled was discouraged, as was buying sucralose-containing canned foods and baked goods.[18][19]
As of 2020, reviews of numerous safety and toxicology studies on sucralose concluded that it is not carcinogenic.[14][15]
History[]
Sucralose was discovered in 1976 by scientists from Tate & Lyle, working with researchers Leslie Hough and Shashikant Phadnis at Queen Elizabeth College (now part of King's College London).[20] While researching novel uses of sucrose and its synthetic derivatives, Phadnis was told to "test" a chlorinated sugar compound. Phadnis thought Hough asked him to "taste" it, so he did.[21] He found the compound to be exceptionally sweet.
Tate & Lyle patented the substance in 1976; as of 2008, the only remaining patents concern specific manufacturing processes.[22]
A Duke University animal study funded by the Sugar Association[23] found evidence that doses of Splenda (containing ~1% sucralose and ~99% maltodextrin by weight) between 100 and 1000 mg/kg BW/day, containing sucralose at 1.1 to 11 mg/kg BW/day, fed to rats reduced fecal microflora, increased the pH level in the intestines, contributed to increases in body weight, and increased levels of P-glycoprotein (P-gp).[24] These effects have not been reported in humans.[4] An expert panel, including scientists from Duke University, Rutgers University, New York Medical College, Harvard School of Public Health, and Columbia University reported in Regulatory Toxicology and Pharmacology that the Duke study was "not scientifically rigorous and is deficient in several critical areas that preclude reliable interpretation of the study results".[25]
Sucralose was first approved for use in Canada in 1991. Subsequent approvals came in Australia in 1993, in New Zealand in 1996, in the United States in 1998, and in the European Union in 2004. By 2008, it had been approved in over 80 countries, including Mexico, Brazil, China, India, and Japan.[26] In 2006, the US Food and Drug Administration amended the regulations for foods to include sucralose as a "non-nutritive sweetener" in food.[27] In May 2008, Fusion Nutraceuticals launched a generic product to the market, using Tate & Lyle patents.
In April 2015 PepsiCo announced that it would be moving from aspartame to sucralose for most of its diet drinks in the US,[28] due to sales of Diet Pepsi falling by more than 5% in the US. PepsiCo says its decision is a commercial one - responding to consumer preferences.
In February 2018 PepsiCo went back to using aspartame in Diet Pepsi because of an 8% drop in sales for the previous year.[29][30]
Chemistry and production[]


Sucralose is a disaccharide composed of 1,6-dichloro-1,6-dideoxyfructose and 4-chloro-4-deoxygalactose. It is synthesized by the selective chlorination of sucrose in a multistep route that substitutes three specific hydroxyl groups with chlorine atoms. This chlorination is achieved by selective protection of one of the primary alcohols as an ester (acetate or benzoate), followed by chlorination with an excess of any of several chlorinating agent to replace the two remaining primary alcohols and one of the secondary alcohols, and finally deprotection by hydrolysis of the ester.[31][32]
Packaging and storage[]
Pure sucralose is sold in bulk, but not in quantities suitable for individual use, although some highly concentrated sucralose–water blends are available online. These concentrates contain one part sucralose for each two parts water. A quarter teaspoon of concentrate substitutes for one cup of sugar. Pure, dry sucralose undergoes some decomposition at elevated temperatures. In solution or blended with maltodextrin, it is slightly more stable. Most products containing sucralose add fillers and additional sweetener to bring the product to the approximate volume and texture of an equivalent amount of sugar.
Effect on caloric content[]
Though sucralose contains no calories, products that contain fillers, such as maltodextrin and/or dextrose, add about 2–4 calories per teaspoon or individual packet, depending on the product, the fillers used, brand, and the intended use of the product.[33] The US Food and Drug Administration (FDA) allows for any product containing fewer than five calories per serving to be labeled as "zero calories".[34]
Environmental effects[]
According to one study, sucralose is digestible by a number of microorganisms and is broken down once released into the environment.[35] However, measurements by the Swedish Environmental Research Institute have shown sewage treatment has little effect on sucralose, which is present in wastewater effluents at levels of several μg/l (ppb).[36] No ecotoxicological effects are known at such levels, but the Swedish Environmental Protection Agency warns a continuous increase in levels may occur if the compound is only slowly degraded in nature. When heated to very high temperatures (over 350 °C or 662 °F) in metal containers, sucralose can produce polychlorinated dibenzo-p-dioxins and other persistent organic pollutants in the resulting smoke.[37]
Sucralose has been detected in natural waters. Studies indicate that this has virtually no impact on the early life development of certain animal species,[38] but the impact on other species remains unknown.
See also[]
References[]
- ^ Merck Index, 11th Edition, 8854.
- ^ Anonymous. Scifinder – Substance Detail for 56038-13-2, 30 October 2010.
- ^ "Gestational Diabetes and Low-Calorie Sweeteners: Answers to Common Questions" (PDF). Food Insight. Archived from the original (PDF) on 9 August 2017. Retrieved 15 May 2015.
- ^ a b Michael A. Friedman, Lead Deputy Commissioner for the FDA, Food Additives Permitted for Direct Addition to Food for Human Consumption; Sucralose Federal Register: 21 CFR Part 172, Docket No. 87F-0086, 3 April 1998
- ^ Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, et al. (July 2017). "Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies". CMAJ. 189 (28): E929–E939. doi:10.1503/cmaj.161390. PMC 5515645. PMID 28716847.
- ^ a b Bannach G, Almeida RR, Lacerda LG, Schnitzler E, Ionashiro M (December 2009). "Thermal stability and thermal decomposition of sucralose" (PDF). Eclética Química. 34 (4): 21–26. doi:10.1590/S0100-46702009000400002.
- ^ de Oliveira DN, de Menezes M, Catharino RR (April 2015). "Thermal degradation of sucralose: a combination of analytical methods to determine stability and chlorinated byproducts". Scientific Reports. 5: 9598. Bibcode:2015NatSR...5E9598D. doi:10.1038/srep09598. PMC 4397539. PMID 25873245.
- ^ A Report on Sucralose from the Food Sanitation Council Archived 15 October 2012 at the Wayback Machine, The Japan Food Chemical Research Foundation
- ^ Food and Drug Administration (March 2006). "Food labeling: health claims; dietary noncariogenic carbohydrate sweeteners and dental caries. Final rule". Federal Register. 71 (60): 15559–64. PMID 16572525.
- ^ Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, Pi-Sunyer FX (December 2003). "Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes". Journal of the American Dietetic Association. 103 (12): 1607–12. doi:10.1016/j.jada.2003.09.021. PMID 14647086.
- ^ FAP 7A3987, 16 August 1996. pp. 1–357. A 12-week study of the effect of sucralose on glucose homeostasis and HbA1c in normal healthy volunteers, Center for Food Safety and Applied Nutrition, U.S. FDA
- ^ Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, Bloom SR (April 2011). "Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects". European Journal of Clinical Nutrition. 65 (4): 508–13. doi:10.1038/ejcn.2010.291. PMID 21245879. S2CID 13051016.
- ^ "Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada" (PDF). Canadian Journal of Diabetes. 32 (Supplement 1): S41. September 2008. Archived from the original (PDF) on 16 May 2012. Retrieved 10 July 2012.
- ^ a b c d e f Magnuson, Bernadene A.; Roberts, Ashley; Nestmann, Earle R. (2017). "Critical review of the current literature on the safety of sucralose". Food and Chemical Toxicology. 106 (Pt A): 324–355. doi:10.1016/j.fct.2017.05.047. ISSN 0278-6915. PMID 28558975.
- ^ a b c d e f g Berry C, Brusick D, Cohen SM, Hardisty JF, Grotz VL, Williams GM (16 November 2016). "Sucralose Non-Carcinogenicity: A Review of the Scientific and Regulatory Rationale". Nutrition and Cancer. 68 (8): 1247–1261. doi:10.1080/01635581.2016.1224366. PMC 5152540. PMID 27652616.
- ^ "Sucralose - FDA Final Rule - Food Additives Permitted for Direct Addition to Food for Human Consumption" (PDF). United States: Food and Drug Administration. Archived from the original (PDF) on 18 October 2012. Retrieved 17 July 2011.
- ^ "Heating food sweetened with sucralose may be harming your health". SlashGear. 13 April 2019. Retrieved 22 June 2020.
- ^ a b Bundesinstitut Für Risikobewertung (9 April 2019). "Harmful compounds might be formed when foods containing the sweetener Sucralose are heated: BfR Opinion No 012/2019 of 9 April 2019" (PDF). Bundesinstitut Für Risikobewertung. doi:10.17590/20190409-142644. Cite journal requires
|journal=(help) - ^ Eisenreich A, Gürtler R, Schäfer B (August 2020). "Heating of food containing sucralose might result in the generation of potentially toxic chlorinated compounds". Food Chemistry. 321: 126700. doi:10.1016/j.foodchem.2020.126700. PMID 32278984. S2CID 215748494.
- ^ "Frequently Asked Questions About Sucralose". Sucralose. Retrieved 20 September 2018.
- ^ Gratzer W (28 November 2002). "5. Light on sweetness: the discovery of aspartame". Eurekas and Euphorias: The Oxford Book of Scientific Anecdotes. Oxford University Press. pp. 32–. Bibcode:2002eueu.book.....G. ISBN 978-0-19-280403-7. Retrieved 1 August 2012.
- ^ "Tate & Lyle loses sucralose patent case". ap-foodtechnology.com.
- ^ Browning L (2 September 2008). "New Salvo in Splenda Skirmish". The New York Times. Retrieved 24 May 2010.
- ^ Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, McLendon RE, Schiffman SS (2008). "Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats". Journal of Toxicology and Environmental Health. Part A. 71 (21): 1415–29. doi:10.1080/15287390802328630. PMID 18800291. S2CID 11909980.
- ^ Daniells S (2 September 2009). "Sucralose safety 'scientifically sound': Expert panel".
- ^ "SPLENDA Brand Sweetener FAQ: Safety & Product Information: What research has been conducted to confirm the safety of SPLENDA". McNeil Nutritionals, LLC. Retrieved 29 August 2015.
- ^ Turner J (3 April 2006). "FDA amends regulations that include sucralose as a non-nutritive sweetener in food" (PDF). FDA Consumer. Retrieved 7 September 2007.
- ^ Roberts M (27 April 2015). "Pepsi to ditch artificial sweetener". BBC News.
- ^ "Diet Pepsi Revamp Leaves 'Aspartame Free' Gamble Behind". Beverage Digest. 16 February 2018. Archived from the original on 4 July 2018. Retrieved 4 July 2018.
- ^ Schultz EJ (16 February 2018). "Reversing Course, Diet Pepsi Goes All-In on Aspartame". Advertising Age.
- ^ Bert Fraser-Reid, 2012, "From Sugar to Splenda: A Personal and Scientific Journey of a Carbohydrate Chemist and Expert Witness," Berlin:Springer, pp. 199-210, and passim, see [1], accessed 2 November 2014.
- ^ U.S. Patent 5,498,709
- ^ Filipic, Martha Chow Line: Sucralose sweet for calorie-counters (for 10/3/04) Ohio State Human Nutrition article on sucralose
- ^ "CFR – Code of Federal Regulations Title 21". U.S. Food and Drug Administration. 1 April 2011. Retrieved 11 March 2012.
- ^ Labare MP, Alexander M (1993). "Biodegradation of sucralose in samples of natural environments". Environmental Toxicology and Chemistry. 12 (5): 797–804. doi:10.1897/1552-8618(1993)12[797:BOSACC]2.0.CO;2.
- ^ "Measurements of Sucralose in the Swedish Screening Program 2007, Part I; Sucralose in surface waters and STP samples" (PDF).
- ^ Dong S, Liu G, Hu J, Zheng M (October 2013). "Polychlorinated dibenzo-p-dioxins and dibenzofurans formed from sucralose at high temperatures". Scientific Reports. 3: 2946. Bibcode:2013NatSR...3E2946D. doi:10.1038/srep02946. PMC 3796739. PMID 24126490.
- ^ Stoddard KI, Huggett DB (October 2014). "Early life stage (ELS) toxicity of sucralose to fathead minnows, Pimephales promelas". Bulletin of Environmental Contamination and Toxicology. 93 (4): 383–7. doi:10.1007/s00128-014-1348-9. PMID 25120258. S2CID 5380255.
Further reading[]
- An extensive account of the history of the discovery of sucralose, and its patent issues, by a professor of chemistry: Fraser-Reid B (2012). From Sugar to Splenda: A Personal and Scientific Journey of a Carbohydrate Chemist and Expert Witness. Springer. ISBN 978-3642227806.
External links[]
| Wikimedia Commons has media related to Sucralose. |
- Sugar substitutes
- Disaccharides
- Food additives
- Organochlorides
- E-number additives