Giardiasis
| Giardiasis | |
|---|---|
| Other names | Beaver fever, giardia |
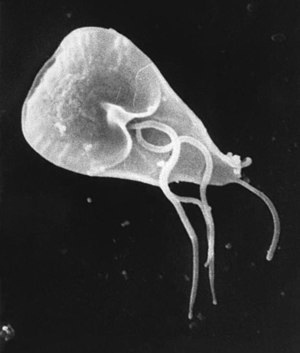 | |
| Giardia cell, SEM | |
| Specialty | Infectious disease, gastroenterology |
| Symptoms | Diarrhea, abdominal pain, weight loss, nausea[1] |
| Usual onset | 1 to 3 weeks after exposure[2] |
| Causes | Giardia duodenalis spread mainly through contaminated food or water[1] |
| Risk factors | Hypogammaglobulinemia |
| Diagnostic method | Stool testing[1] |
| Differential diagnosis | Irritable bowel syndrome[1] |
| Prevention | Improved sanitation[1] |
| Treatment | Antiprotozoal medications |
| Medication | Tinidazole, metronidazole[1] |
| Frequency | Up to 7% (developed world), up to 30% (developing world)[1] |
Giardiasis is a parasitic disease caused by Giardia duodenalis (also known as G. lamblia and G. intestinalis).[3] About 10% of those infected have no symptoms.[1] Individuals who experience symptoms may have diarrhea, abdominal pain, and weight loss.[1] Less common symptoms include vomiting and blood in the stool.[1] Symptoms usually begin 1 to 3 weeks after exposure and without treatment, may last up to six weeks.[2]
Giardia usually spreads when Giardia duodenalis cysts within feces contaminate food or water, which is later consumed orally.[1] The disease can also spread between people and through other animals.[1] Factors that increase possible contamination include travelling in the developing world, changing diapers, consuming raw food, and owning a dog.[1] Cysts may survive for nearly three months in cold water.[1] Giardia is diagnosed via stool tests.[1]
Prevention may be improved through proper hygiene practices.[1] Asymptomatic cases often do not need treatment.[1] When symptoms are present, treatment is typically provided with either tinidazole or metronidazole.[1] Infection may cause one to become lactose intolerant and as a result, it is recommended to temporarily avoid lactose following an infection.[1] Resistance to treatment may occur in some patients.[1]
Giardia is one of the most common parasitic human diseases globally.[3] In 2013, there were approximately 280 million people worldwide with symptomatic cases of giardiasis.[3] Infection rates are as high as 7% in the developed world and 30% in the developing world.[1] The World Health Organization classifies Giardia as a neglected disease.[1]
Signs and symptoms[]
Symptoms vary from none to severe diarrhea with poor absorption of nutrients.[4] The cause of this wide range in severity of symptoms is not fully known but the intestinal flora of the infected host may play a role.[5][6] Diarrhea is less likely to occur in people from developing countries.[5]
Symptoms typically develop 9–15 days after exposure,[7] but may occur as early as one day.[4] The most common and prominent symptom is chronic diarrhea which can occur for weeks or months if untreated.[8][9] Diarrhea is often greasy and foul-smelling, with a tendency to float.[8][10] This characteristic diarrhea is often accompanied by a number of other symptoms, including gas, abdominal cramps, and nausea or vomiting.[8][10] Some people also experience symptoms outside of the gastrointestinal tract such as itchy skin, hives, and swelling of the eyes and joints, although these are less common.[10] Despite its nickname "beaver fever",[11] fever occurs in only about 15% of people.[12]
Prolonged disease is often characterized by diarrhea, along with malabsorption of nutrients in the intestine.[8] This malabsorption results in fatty stools, substantial weight loss, and fatigue.[8] Additionally, those suffering from giardiasis often have difficulty absorbing lactose, vitamin A, folate, and vitamin B12.[9][10] In children, prolonged giardiasis can cause failure to thrive and may impair mental development.[8][9] Symptomatic infections are well recognized as causing lactose intolerance,[13] which, while usually temporary, may become permanent.[14][15]
Cause[]
Giardiasis is caused by the protozoan Giardia duodenalis.[16] The infection occurs in many animals including beavers (hence its nickname), as well as cows, other rodents, and sheep.[16] Animals are believed to play a role in keeping infections present in an environment.[16]
G. duodenalis has been sub-classified into eight genetic assemblages (designated A–H).[17] Genotyping of G. duodenalis isolated from various hosts has shown that assemblages A and B infect the largest range of host species, and appear to be the main and possibly only G. duodenalis assemblages that infect humans.[17][18]
Risk factors[]
According to the CDC, those at greatest risk are:
- People in childcare settings
- People who are in close contact with someone who has the disease
- Travelers within areas that have poor sanitation
- People who have contact with feces during sexual activity
- Backpackers or campers who drink untreated water from springs, lakes, or rivers
- Swimmers who swallow water from swimming pools, hot tubs, interactive fountains, or untreated recreational water from springs, lakes, or rivers
- People who get their household water from a shallow well
- People with weakened immune systems
- People who have contact with infected animals or animal environments contaminated with feces[19]
In the United States, giardiasis occurs more often during the summer.[16] This is believed to be due to a greater amount of time spent on outdoor activities and traveling in the wilderness.[16]
Transmission[]
Giardiasis is transmitted via the fecal-oral route with the ingestion of cysts.[7] Primary routes are personal contact and contaminated water and food.[7] The cysts can stay infectious for up to three months in cold water.[16]
Many people with Giardia infections have no or few symptoms.[20] They may, however, still spread the disease.[20]
Pathophysiology[]

The life cycle of Giardia consists of a cyst form and a trophozoite form.[6] The cyst form is infectious and once it has found a host, transforms into the trophozoite form.[6] This trophozoite attaches to the intestinal wall and replicates within the gut.[6] As trophozoites continue along the gastrointestinal tract, they convert back to their cyst form which is then excreted with feces.[1] Ingestion of only a few of these cysts is needed to generate infection in another host.[21]
Infection with Giardia results in decreased expression of brush border enzymes, morphological changes to the microvillus, increased intestinal permeability, and programmed cell death of small intestinal epithelial cells.[22] Both trophozoites and cysts are contained within the gastrointestinal tract and do not invade beyond it.[23]
The attachment of trophozoites causes villous flattening and inhibition of enzymes that break down disaccharide sugars in the intestines.[5][22] Ultimately, the community of microorganisms that lives in the intestine may overgrow and may be the cause of further symptoms, though this idea has not been fully investigated. The alteration of the villi leads to an inability of nutrient and water absorption from the intestine, resulting in diarrhea, one of the predominant symptoms.[22] In the case of asymptomatic giardiasis, there can be malabsorption with or without histological changes to the small intestine. The degree to which malabsorption occurs in symptomatic and asymptomatic cases is highly varied.[citation needed]
The species Giardia intestinalis uses enzymes that break down proteins to attack the villi of the brush border and appears to increase crypt cell proliferation and crypt length of crypt cells existing on the sides of the villi. On an immunological level, activated host T lymphocytes attack endothelial cells that have been injured in order to remove the cell.[5] This occurs after the disruption of proteins that connect brush border endothelial cells to one another.[22] The result is increased intestinal permeability.[22]
There appears to be a further increase in programmed enterocyte cell death by Giardia intestinalis, which further damages the intestinal barrier and increases permeability.[22] There is significant upregulation of the programmed cell death cascade by the parasite, and, furthermore, substantial downregulation of the anti-apoptotic protein Bcl-2 and upregulation of the proapoptotic protein Bax.[5] These connections suggest a role of caspase-dependent apoptosis in the pathogenesis of giardiasis.[5]
Giardia protects its own growth by reducing the formation of the gas nitric oxide by consuming all local arginine, which is the amino acid necessary to make nitric oxide.[5] Arginine starvation is known to be a cause of programmed cell death, and local removal is a strong apoptotic agent.[24]
Host Defence[]
Host defense against Giardia consists of natural barriers, production of nitric oxide, and activation of the innate and adaptive immune systems.
Natural barriers[]
Natural barriers defend against parasite entering the host's body. Natural barriers consist of mucus layers, bile salt, proteases, and lipases. Additionally, peristalsis and the renewal of enterocytes provide further protection against parasites.[25][26]
Nitric oxide (NO) production[]
Nitric oxide does not kill the parasite, but it inhibits the growth of trophozoites as well as excystation and encystation.[27][28]
Innate immune system[]
Lectin pathway of complement[]
The lectin pathway of complement is activated by mannose-binding lectin (MBL) which binds to N-acetylglucosamine. N-acetylglucosamine is a ligand for MBL and is present on the surface of Giardia.[29]
The classical pathway of complement[]
The classical pathway of complement is activated by antibodies specific against Giardia.
Adaptive immune system[]
Antibodies[]
Antibodies inhibit parasite replication and also induce parasite death via the classical pathway of complement.
Infection with Giardia typically results in a strong antibody response against the parasite. While IgG is made in significant amounts, IgA is believed to be more important in parasite control. IgA is the most abundant isotype in intestinal secretions, and it is also the dominant isotype in mother’s milk. Antibodies in mother’s milk protect children against giardiasis (passive immunization).[30]
T cells[]
The major aspect of adaptive immune responses is the T cell response. Giardia is an extracellular pathogen. Therefore CD4+ helper T cells are primarily responsible for this protective effect.[31]
One role of helper T cells is to promote antibody production and isotype switching. Other roles include cytokine production (Il-4,IL-9) to help recruit other effector cells of the immune response.[31][32]
Diagnosis[]
- According to the CDC, detection of antigens on the surface of organisms in stool specimens is the current test of choice for diagnosis of giardiasis and provides increased sensitivity over more common microscopy techniques.[33]
- A trichrome stain of preserved stool is another method used to detect Giardia.[34]
- Microscopic examination of the stool can be performed for diagnosis.[1] This method is not preferred, however, due to inconsistent shedding of trophozoites and cysts in infected hosts.[1] Multiple samples over a period of time, typically one week, must be examined.[1]
- The uses a gelatin capsule with an attached thread. One end is attached to the inner aspect of the host's cheek, and the capsule is swallowed. Later, the thread is withdrawn and shaken in saline to release trophozoites which can be detected with a microscope. The sensitivity of this test is low, however, and is not routinely used for diagnosis.[35]
- Immunologic enzyme-linked immunosorbent assay (ELISA) testing may be used for diagnosis.[36] These tests are capable of a 90% detection rate or more.[36]
Although hydrogen breath tests indicate poorer rates of carbohydrate absorption in those asymptomatically infected, such tests are not diagnostic of infection.[37] Serological tests are not helpful in diagnosis.[1]
Prevention[]
The CDC recommends hand-washing and avoiding potentially contaminated food and untreated water.[38]
Boiling water contaminated with Giardia effectively kills infectious cysts.[39] Chemical disinfectants or filters may be used.[40][41] Iodine-based disinfectants are preferred over chlorination as the latter is ineffective at destroying cysts.[42][43]
Although the evidence linking the drinking of water in the North American wilderness and giardiasis has been questioned, a number of studies raise concern.[44] Most if not all CDC verified backcountry giardiasis outbreaks have been attributed to water. Surveillance data (for 2013 and 2014) reports six outbreaks (96 cases) of waterborne giardiasis contracted from rivers, streams or springs[45] and less than 1% of reported giardiasis cases are associated with outbreaks.[46]
Person-to-person transmission accounts for the majority of Giardia infections, and is usually associated with poor hygiene and sanitation. Giardia is often found on the surface of the ground, in the soil, in undercooked foods, and in water, and on hands that have not been properly cleaned after handling infected feces.[47] Water-borne transmission is associated with the ingestion of contaminated water. In the U.S., outbreaks typically occur in small water systems using inadequately treated surface water. Venereal transmission happens through fecal-oral contamination. Additionally, diaper changing and inadequate handwashing are risk factors for transmission from infected children. Lastly, food-borne epidemics of Giardia have developed through the contamination of food by infected food-handlers.[48]
Treatment[]
Treatment is not always necessary as the infection usually resolves on its own.[5] However, if the illness is acute or symptoms persist and medications are needed to treat it, a nitroimidazole medication is used such as metronidazole, tinidazole, secnidazole or ornidazole.[7]
The World Health Organization and Infectious Disease Society of America recommend metronidazole as first line therapy.[49][50] The US CDC lists metronidazole, tinidazole, and nitazoxanide as effective first-line therapies;[51] of these three, only nitazoxanide and tinidazole are approved for the treatment of giardiasis by the US FDA.[52][53][54] A meta-analysis done by the Cochrane Collaboration found that compared to the standard of metronidazole, albendazole had equivalent efficacy while having fewer side effects, such as gastrointestinal or neurologic issues.[55] Other meta-analyses have reached similar conclusions.[56] Both medications need a five to 10 day long course; albendazole is taken once a day, while metronidazole needs to be taken three times a day. The evidence for comparing metronidazole to other alternatives such as mebendazole, tinidazole or nitazoxanide was felt to be of very low quality.[55] While tinidazole has side effects and efficacy similar to those of metronidazole, it is administered with a single dose.[20]
Resistance has been seen clinically to both nitroimidazoles and albendazole, but not nitazoxanide, though nitazoxanide resistance has been induced in research laboratories.[21][57] The exact mechanism of resistance to all of these medications is not well understood.[57] In the case of nitroimidazole-resistant strains of Giardia, other drugs are available which have showed efficacy in treatment including quinacrine, nitazoxanide, bacitracin zinc, furazolidone and paromomycin.[20] Mepacrine may also be used for refractory cases.[21]
Probiotics, when given in combination with the standard treatment, has been shown to assist with clearance of Giardia.[58]
During pregnancy, paromomycin is the preferred treatment drug because of its poor intestinal absorption, resulting in less exposure to the fetus.[59] Alternatively, metronidazole can be used after the first trimester as there has been wide experience in its use for trichomonas in pregnancy.[20][60]
Vaccine[]
There are no vaccines for humans yet, however there are several vaccine candidates in development. They are targeting: recombinant proteins, DNA vaccine, variant-specific surface proteins (VSP), cyst wall proteins (CWP), giadins and enzymes.[61]
At present, one commercially available vaccine exists – GiardiaVax. It is a vaccine for veterinary use only - for dogs and cats. GiardiaVax should promote production of specific antibodies.[62]
Prognosis[]
In people with a properly functioning immune system, infection may resolve without medication.[5] A small portion, however, develop a chronic infection.[5] People with an impaired immune system are at higher risk of chronic infection.[5] Medication is an effective cure for nearly all people although there is growing drug-resistance.[1][63][21]
Children with chronic giardiasis are at risk for failure to thrive as well as more long-lasting sequelae such as growth stunting.[64] Up to half of infected people develop a temporary lactose intolerance leading symptoms that may mimic a chronic infection.[1] Some people experience post-infectious irritable bowel syndrome after the infection has cleared.[5] Giardiasis has also been implicated in the development of food allergies.[5] This is thought to be due to its effect on intestinal permeability.[5]
Epidemiology[]

In some developing countries Giardia is present in 30% of the population.[16] In the United States it is estimated that it is present in 3–7% of the population.[16]
The number of reported cases in the United States in 2018 was 15,584.[65] All states that classify giardiasis as a notifiable disease had cases of giardiasis.[65] The states of Illinois, Kentucky, Mississippi, North Carolina, Oklahoma, Tennessee, Texas, and Vermont did not notify the Center for Disease Control regarding cases in 2018.[65] The states with the highest number of cases in 2018 were California, New York, Florida, and Wisconsin.[65] There are seasonal trends associated with giardiasis.[66] July, August, and September are the months with the highest incidence of giardiasis in the United States.[67]
In the ECDC's (European Centre for Disease Prevention Control) annual epidemiological report containing 2014 data, 17,278 confirmed giardiasis cases were reported by 23 of the 31 countries that are members of the EU/EEA.[68] Germany reported the highest number at 4,011 cases.[68] Following Germany, the UK reported 3,628 confirmed giardiasis cases. Together, this accounts for 44% of total reported cases.[68]
Research[]
Some intestinal parasitic infections may play a role in irritable bowel syndrome[69] and other long-term sequelae such as chronic fatigue syndrome.[70][71] The mechanism of transformation from cyst to trophozoites has not been characterized[6] but may be helpful in developing drug targets for treatment-resistant Giardia. The interaction between Giardia and host immunity, internal flora, and other pathogens is not well understood.[5]
The main congress about giardiasis is the "International Giardia and Cryptosporidium Conference" (IGCC). A summary of results presented at the most recent edition (2019, in Rouen, France) is available.[72]
Other animals[]
In both dogs and cats, giardiasis usually responds to metronidazole and fenbendazole. Metronidazole in pregnant cats can cause developmental malformations.[73] Many cats dislike the taste of febendazole.[73] Giardiasis has been shown to decrease weight in livestock.[5]
References[]
- ^ Jump up to: a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac Minetti C, Chalmers RM, Beeching NJ, Probert C, Lamden K (October 2016). "Giardiasis" (PDF). BMJ. 355: i5369. doi:10.1136/bmj.i5369. PMID 27789441. S2CID 220092781.
- ^ Jump up to: a b "Parasites - Giardia". CDC. 21 July 2015. Archived from the original on 17 November 2016. Retrieved 17 November 2016.
- ^ Jump up to: a b c Esch KJ, Petersen CA (January 2013). "Transmission and epidemiology of zoonotic protozoal diseases of companion animals". Clinical Microbiology Reviews. 26 (1): 58–85. doi:10.1128/CMR.00067-12. PMC 3553666. PMID 23297259.
- ^ Jump up to: a b "Giardiasis". cdc.gov. November 29, 2013. Archived from the original on January 15, 2016. Retrieved 1 Jan 2016.
- ^ Jump up to: a b c d e f g h i j k l m n o p Cotton JA, Beatty JK, Buret AG (August 2011). "Host parasite interactions and pathophysiology in Giardia infections". International Journal for Parasitology. 41 (9): 925–33. doi:10.1016/j.ijpara.2011.05.002. PMID 21683702.
- ^ Jump up to: a b c d e Einarsson E, Ma'ayeh S, Svärd SG (December 2016). "An up-date on Giardia and giardiasis". Current Opinion in Microbiology. 34: 47–52. doi:10.1016/j.mib.2016.07.019. PMID 27501461.
- ^ Jump up to: a b c d Barry MA, Weatherhead JE, Hotez PJ, Woc-Colburn L (April 2013). "Childhood parasitic infections endemic to the United States". Pediatric Clinics of North America. 60 (2): 471–85. doi:10.1016/j.pcl.2012.12.011. PMID 23481112.
- ^ Jump up to: a b c d e f Despommier DD, Griffin DO, Gwadz RW, Hotez PJ, Knirsch CA. "III. Eukaryotic Parasites". Parasitic Diseases (6 ed.). NY: Parasites Without Borders. pp. 11–17. Retrieved 11 July 2018.
- ^ Jump up to: a b c Robertson LJ, Hanevik K, Escobedo AA, Mørch K, Langeland N (February 2010). "Giardiasis--why do the symptoms sometimes never stop?". Trends in Parasitology. 26 (2): 75–82. doi:10.1016/j.pt.2009.11.010. PMID 20056486.
- ^ Jump up to: a b c d "Giardia - Illness & Symptoms". CDC. Retrieved 11 July 2018.
- ^ "Giardiasis (beaver fever)". New York State Department of Health. October 2011. Archived from the original on 11 May 2015. Retrieved 21 June 2015.
- ^ Guerrant, Richard L.; Walker, David H.; Weller, Peter F. (2011). Tropical infectious diseases : principles, pathogens and practice (3rd ed.). Edinburgh: Saunders/Elsevier. pp. 623. ISBN 9781437737776. OCLC 722800379.
- ^ Pettoello Mantovani M, Guandalini S, Ecuba P, Corvino C, di Martino L (October 1989). "Lactose malabsorption in children with symptomatic Giardia lamblia infection: feasibility of yogurt supplementation". Journal of Pediatric Gastroenterology and Nutrition. 9 (3): 295–300. doi:10.1097/00005176-198910000-00006. PMID 2614615. S2CID 32254397.
- ^ Wolfe MS (September 1975). "Giardiasis". JAMA. 233 (13): 1362–5. doi:10.1001/jama.233.13.1362. PMID 1174208.
- ^ Vega-Franco L, Meza C, Romero JL, Alanis SE, Meijerink J (1987). "Breath hydrogen test in children with giardiasis". Journal of Pediatric Gastroenterology and Nutrition. 6 (3): 365–8. doi:10.1097/00005176-198705000-00010. PMID 3430245. S2CID 20733304.
- ^ Jump up to: a b c d e f g h Auerbach, Paul S. (2012). Wilderness medicine (6th ed.). Philadelphia, PA: Elsevier/Mosby. pp. Chapter 68. ISBN 9781437716788.
- ^ Jump up to: a b Heyworth MF (2016). "Giardia duodenalis genetic assemblages and hosts". Parasite. 23: 13. doi:10.1051/parasite/2016013. PMC 4794627. PMID 26984116. Archived from the original on 2017-09-10.

- ^ Lalle M, Hanevik K (2018-10-24). "Treatment-refractory giardiasis: challenges and solutions". Infection and Drug Resistance. 11: 1921–1933. doi:10.2147/idr.s141468. PMC 6207226. PMID 30498364.
- ^ CDC (July 15, 2015). "Sources of Infection & Risk Factors". Parasites – Giardia. Archived from the original on September 7, 2017.
- ^ Jump up to: a b c d e Gardner TB, Hill DR (January 2001). "Treatment of giardiasis". Clinical Microbiology Reviews. 14 (1): 114–28. doi:10.1128/CMR.14.1.114-128.2001. PMC 88965. PMID 11148005.
- ^ Jump up to: a b c d Carter ER, Nabarro LE, Hedley L, Chiodini PL (January 2018). "Nitroimidazole-refractory giardiasis: a growing problem requiring rational solutions". Clinical Microbiology and Infection. 24 (1): 37–42. doi:10.1016/j.cmi.2017.05.028. PMID 28624613.
- ^ Jump up to: a b c d e f Buret AG (September 2008). "Pathophysiology of enteric infections with Giardia duodenalius". Parasite. 15 (3): 261–5. doi:10.1051/parasite/2008153261. PMID 18814692.
- ^ Bartelt LA, Sartor RB (2015-05-26). "Advances in understanding Giardia: determinants and mechanisms of chronic sequelae". F1000prime Reports. 7 (62): 62. doi:10.12703/P7-62. PMC 4447054. PMID 26097735.
- ^ Muhkerjee, Sandeep. "Giardiasis". Medscape Reference. Archived from the original on 17 November 2012. Retrieved 21 November 2012.
- ^ Eckmann, Lars (May 2003). "Mucosal defences against Giardia". Parasite Immunology. 25 (5): 259–270. doi:10.1046/j.1365-3024.2003.00634.x. ISSN 0141-9838.
- ^ Thompson, R. C. Andrew (July 2011). "Giardia infections". Oxford Medicine Online. doi:10.1093/med/9780198570028.003.0052.
- ^ Eckmann, Lars; Laurent, Fabrice; Langford, T. Dianne; Hetsko, Michael L.; Smith, Jennifer R.; Kagnoff, Martin F.; Gillin, Frances D. (2000-02-01). "Nitric Oxide Production by Human Intestinal Epithelial Cells and Competition for Arginine as Potential Determinants of Host Defense Against the Lumen-Dwelling PathogenGiardia lamblia". The Journal of Immunology. 164 (3): 1478–1487. doi:10.4049/jimmunol.164.3.1478. ISSN 0022-1767.
- ^ Li, Erqiu; Zhou, Ping; Singer, Steven M. (2005-12-19). "Neuronal Nitric Oxide Synthase Is Necessary for Elimination of Giardia lamblia Infections in Mice". The Journal of Immunology. 176 (1): 516–521. doi:10.4049/jimmunol.176.1.516. ISSN 0022-1767.
- ^ Evans-Osses, Ingrid; Ansa-Addo, Ephraim A.; Inal, Jameel M.; Ramirez, Marcel I. (May 2010). "Involvement of lectin pathway activation in the complement killing of Giardia intestinalis". Biochemical and Biophysical Research Communications. 395 (3): 382–386. doi:10.1016/j.bbrc.2010.04.025. ISSN 0006-291X.
- ^ Tellez, Aleyda; Winiecka-krusnell, Jadwiga; Paniagua, Margarita; Linder, Ewert (May 2003). "Antibodies in Mother's Milk Protect Children Against Giardiasis". Scandinavian Journal of Infectious Diseases. 35 (5): 322–325. doi:10.1080/00365540310008041. ISSN 0036-5548.
- ^ Jump up to: a b Singer, Steven M.; Nash, Theodore E. (2000-01-01). "T-Cell-Dependent Control of Acute Giardia lamblia Infections in Mice". Infection and Immunity. 68 (1): 170–175. doi:10.1128/iai.68.1.170-175.2000. ISSN 1098-5522.
- ^ Scott, Kevin G.-E.; Yu, Linda C. H.; Buret, André G. (June 2004). "Role of CD8+ and CD4+ T Lymphocytes in Jejunal Mucosal Injury during Murine Giardiasis". Infection and Immunity. 72 (6): 3536–3542. doi:10.1128/iai.72.6.3536-3542.2004. ISSN 0019-9567.
- ^ "Archived copy". Archived from the original on 2017-06-17. Retrieved 2017-09-09.CS1 maint: archived copy as title (link)
- ^ "Ova and Parasite Exam, Fecal (Immunocompromised or Travel History)". Archived from the original on 2014-10-29. Retrieved 2014-10-29.
- ^ Hooshyar H, Rostamkhani P, Arbabi M, Delavari M (2019). "Giardia lamblia infection: review of current diagnostic strategies". Gastroenterology and Hepatology from Bed to Bench. 12 (1): 3–12. PMC 6441489. PMID 30949313.
- ^ Jump up to: a b Rosenblatt JE, Sloan LM, Schneider SK (May–June 1993). "Evaluation of an enzyme-linked immunosorbent assay for the detection of Giardia lamblia in stool specimens". Diagnostic Microbiology and Infectious Disease. 16 (4): 337–41. doi:10.1016/0732-8893(93)90086-M. PMID 8495591.
- ^ Moya-Camarena SY, Sotelo N, Valencia ME (March 2002). "Effects of asymptomatic Giardia intestinalis infection on carbohydrate absorption in well-nourished Mexican children". The American Journal of Tropical Medicine and Hygiene. 66 (3): 255–9. doi:10.4269/ajtmh.2002.66.255. PMID 12139217.
- ^ "Parasites - Giardia, Prevention & Control". Centers for Disease Control and Prevention. CDC. Archived from the original on 30 April 2015. Retrieved 26 April 2015.
- ^ "Emergency Disinfection of Drinking Water". United States Environment Protection Agency. 2013-02-20. Archived from the original on 23 June 2015. Retrieved 21 June 2015. Retrieved 24 February 2011
- ^ Betancourt WQ, Rose JB (December 2004). "Drinking water treatment processes for removal of Cryptosporidium and Giardia". Veterinary Parasitology. 126 (1–2): 219–34. doi:10.1016/j.vetpar.2004.09.002. PMID 15567586.
- ^ Exner M, Gornik V (July 2004). "[Parasitic zoonoses transmitted by drinking water. Giardiasis and cryptosporidiosis]". Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 47 (7): 698–704. doi:10.1007/s00103-004-0863-y. PMID 15254826.
- ^ Dunn, Noel; Juergens, Andrew L. (2019), "Giardiasis", StatPearls, StatPearls Publishing, PMID 30020611, retrieved 2019-10-31
- ^ Ongerth JE, Johnson RL, Macdonald SC, Frost F, Stibbs HH (December 1989). "Back-country water treatment to prevent giardiasis". American Journal of Public Health. 79 (12): 1633–7. doi:10.2105/ajph.79.12.1633. PMC 1349767. PMID 2817191.
- ^ Julia E. Painter, PhD; Julia W. Gargano, PhD; Sarah A. Collier, MPH; Jonathan S. Yoder, MPH (2015). "Giardiasis Surveillance—United States, 2011–2012". MMWR Supplements. 64 (Suppl 3): 15–25. PMID 25928582. Retrieved 31 March 2018.
- ^ McClung RP, Roth DM, Vigar M, Roberts VA, Kahler AM, Cooley LA, et al. (November 2017). "Waterborne Disease Outbreaks Associated With Environmental and Undetermined Exposures to Water - United States, 2013-2014". MMWR. Morbidity and Mortality Weekly Report. 66 (44): 1222–1225. doi:10.15585/mmwr.mm6644a4. PMC 5679586. PMID 29120997.
- ^ "Giardiasis Surveillance — United States, 2009–2010". www.cdc.gov.
- ^ CDC Giardia 2011
- ^ Giardiasis at eMedicine
- ^ Guerrant RL, Van Gilder T, Steiner TS, Thielman NM, Slutsker L, Tauxe RV, et al. (February 2001). "Practice guidelines for the management of infectious diarrhea" (PDF). Clinical Infectious Diseases. 32 (3): 331–51. doi:10.1086/318514. PMID 11170940. Archived (PDF) from the original on February 10, 2016.
- ^ "Chapter 7.5.4 Continuing Diarrhoea | ICHRC". www.ichrc.org. Archived from the original on 2016-01-29. Retrieved 2016-01-09.
- ^ "Giardia: Treatment". United States Centers for Disease Control and Prevention. 21 July 2015. Archived from the original on 24 December 2015. Retrieved 10 January 2016.
Several drugs can be used to treat Giardia infection. Effective treatments include metronidazole, tinidazole, and nitazoxanide1. Alternatives to these medications include paromomycin, quinacrine, and furazolidone1,2.
- ^ "Nitazoxanide Prescribing Information" (PDF). Romark Pharmaceuticals. August 2013. pp. 1–5. Archived from the original (PDF) on 16 January 2016. Retrieved 3 January 2016.
- ^ "Metronidazole Prescribing Information" (PDF). United States Food and Drug Administration. Pfizer. June 2015. pp. 6–7. Archived (PDF) from the original on 4 March 2016. Retrieved 10 January 2016.
- ^ "Tinidazole Prescribing Informatiuon" (PDF). United States Food and Drug Administration. Mission Pharma. May 2007. p. 1. Archived (PDF) from the original on 4 March 2016. Retrieved 10 January 2016.
- ^ Jump up to: a b Granados CE, Reveiz L, Uribe LG, Criollo CP (December 2012). "Drugs for treating giardiasis". The Cochrane Database of Systematic Reviews. 12: CD007787. doi:10.1002/14651858.cd007787.pub2. PMC 6532677. PMID 23235648.
- ^ Solaymani-Mohammadi S, Genkinger JM, Loffredo CA, Singer SM (May 2010). Keiser J (ed.). "A meta-analysis of the effectiveness of albendazole compared with metronidazole as treatments for infections with Giardia duodenalis". PLOS Neglected Tropical Diseases. 4 (5): e682. doi:10.1371/journal.pntd.0000682. PMC 2867942. PMID 20485492.
- ^ Jump up to: a b Leitsch D (2015-07-07). "Giardia lamblia". Current Tropical Medicine Reports. 2 (3): 128–135. doi:10.1007/s40475-015-0051-1. PMC 4523694. PMID 26258002.
- ^ Lalle, Marco; Hanevik, Kurt (2018-10-24). "Treatment-refractory giardiasis: challenges and solutions". Infection and Drug Resistance. 11: 1921–1933. doi:10.2147/IDR.S141468. ISSN 1178-6973. PMC 6207226. PMID 30498364.
- ^ Farthing MJ (August 2006). "Treatment options for the eradication of intestinal protozoa". Nature Clinical Practice. Gastroenterology & Hepatology. 3 (8): 436–45. doi:10.1038/ncpgasthep0557. PMID 16883348. S2CID 19657328.
- ^ Workowski KA, Bolan GA (June 2015). "Sexually transmitted diseases treatment guidelines, 2015". MMWR. Recommendations and Reports. 64 (RR-03): 1–137. PMC 5885289. PMID 26042815.
- ^ Davids, Barbara J.; Liu, Ching M.; Hanson, Elaine M.; Le, Christine H. Y.; Ang, Jonathan; Hanevik, Kurt; Fischer, Marvin; Radunovic, Matej; Langeland, Nina; Ferella, Marcela; Svärd, Staffan G. (2019-04-08). "Identification of Conserved Candidate Vaccine Antigens in the Surface Proteome of Giardia lamblia". Infection and Immunity. 87 (6). doi:10.1128/iai.00219-19. ISSN 0019-9567.
- ^ "Immunotherapeutic agent derived from Mycobacterium vaccae for vaccination against persistent microorganisms causing tuberculosis or leprosy". Vaccine. 4 (1): 65. March 1986. doi:10.1016/0264-410x(86)90126-x. ISSN 0264-410X.
- ^ Kasper, Dennis L.; Larry Jameson, J.; Hauser, Stephen L.; Loscalzo, Joseph; Fauci, Anthony S.; Longo, Dan L. (2015-04-08). Harrison's principles of internal medicine. Kasper, Dennis L.,, Fauci, Anthony S., 1940-, Hauser, Stephen L.,, Longo, Dan L. (Dan Louis), 1949-, Jameson, J. Larry,, Loscalzo, Joseph (19th ed.). New York. ISBN 9780071802154. OCLC 893557976.
- ^ Donowitz JR, Alam M, Kabir M, Ma JZ, Nazib F, Platts-Mills JA, et al. (September 2016). "A Prospective Longitudinal Cohort to Investigate the Effects of Early Life Giardiasis on Growth and All Cause Diarrhea". Clinical Infectious Diseases. 63 (6): 792–7. doi:10.1093/cid/ciw391. PMC 4996141. PMID 27313261.
- ^ Jump up to: a b c d "TABLE 2f. Annual reported cases of notifiable diseases, by region and reporting area - - United States and U.S. Territories, 2018". wonder.cdc.gov. Retrieved 2019-11-13.
- ^ Painter, Julia E.; Gargano, Julia W.; Collier, Sarah A.; Yoder, Jonathan S. (2015-05-01). "Giardiasis surveillance -- United States, 2011-2012". MMWR Supplements. 64 (3): 15–25. ISSN 2380-8942. PMID 25928582.
- ^ Jonathan S. Yoder, MPH; Julia W. Gargano, PhD; Ryan M. Wallace, DVM; Michael J. Beach, PhD (2012). "Giardiasis Surveillance-United States, 2009-2010". Morbidity and Mortality Weekly Report. Surveillance Summaries. Centers of Disease Control and Prevention. 61 (5): 13–23. PMID 22951494. Retrieved 30 November 2017.
- ^ Jump up to: a b c "Giardiasis- Annual Epidemiological Report 2016". European Centre for Disease Prevention and Control. European Centre for Disease Prevention and Control. 2017-01-30. Retrieved 30 November 2017.
- ^ Stark D, van Hal S, Marriott D, Ellis J, Harkness J (January 2007). "Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis". International Journal for Parasitology. 37 (1): 11–20. doi:10.1016/j.ijpara.2006.09.009. PMID 17070814.
- ^ Hanevik K, Wensaas KA, Rortveit G, Eide GE, Mørch K, Langeland N (November 2014). "Irritable bowel syndrome and chronic fatigue 6 years after giardia infection: a controlled prospective cohort study". Clinical Infectious Diseases. 59 (10): 1394–400. doi:10.1093/cid/ciu629. PMC 4207419. PMID 25115874.
- ^ Quote: "for unclear reasons, chronic sequelae, including post-infectious irritable bowel syndrome, chronic fatigue [..], malnutrition [..], cognitive impairment [..], and extra-intestinal manifestations (such as food allergy, urticaria, reactive arthritis, and inflammatory ocular manifestations), can develop and possibly persist beyond detectable parasite shedding". Quoted from: Bartelt LA, Sartor RB (2015). "Advances in understanding Giardia: determinants and mechanisms of chronic sequelae". F1000prime Reports (Review). 7: 62. doi:10.12703/P7-62. PMC 4447054. PMID 26097735.
- ^ Buret, André G.; Cacciò, Simone M.; Favennec, Loïc; Svärd, Staffan (2020). "Update on Giardia: Highlights from the seventh International Giardia and Cryptosporidium Conference". Parasite. 27: 49. doi:10.1051/parasite/2020047. ISSN 1776-1042. PMC 7425178. PMID 32788035.

- ^ Jump up to: a b Eldredge, Debra M. (2008). Cat Owner's Home Veterinary Handbook. Howell Book House. p. 67.
External links[]
| Classification | |
|---|---|
| External resources |
- Protozoal diseases
- Waterborne diseases
- Animal diseases
- Neglected tropical diseases
- Tropical diseases
- Zoonoses
- Feces