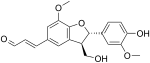
Balanophonin
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C20H20O6 | |
| Molar mass | 356.37 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Balanophonin is a neo-lignan. It is a bioactive compound which can be isolated from Dipteryx odorata[2] and Balanophora japonica.[3]
References[]
- ^ "KNApSAcK Metabolite Information - C00033656". www.knapsackfamily.com.
- ^ Jang, DS; Park, EJ; Hawthorne, ME; Vigo, JS; Graham, JG; Cabieses, F; Santarsiero, BD; Mesecar, AD; Fong, HH; Mehta, RG; Pezzuto, JM; Kinghorn, AD (2003). "Potential cancer chemopreventive constituents of the seeds of Dipteryx odorata (tonka bean)". J Nat Prod. 66 (5): 583–7. doi:10.1021/np020522n. PMID 12762787.
- ^ Balanophonin, a new neo-lignan from Balanophora japonica Makino, Haruna Mitsumasa, Koube Tomoko, Ito Kazuo and Murata Hiroyuki, Chemical & Pharmaceutical Bulletin, 25-04-1982, 30(4), pages 1525-1527 (abstract)
External links[]
- http://www.chemfaces.com/natural/Balanophonin-CFN99295.html
- http://www.biocrick.com/Balanophonin-BCN6072.html
Categories:
- Lignans
- Aromatic compound stubs