Electrolysis of water


Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas by a process called electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remixed with the oxygen to create oxyhydrogen gas, which is used in welding and other applications.
Sometimes called water splitting, electrolysis requires a minimum potential difference of 1.23 volts.
History[]
Jan Rudolph Deiman and Adriaan Paets van Troostwijk used, in 1789, an electrostatic machine to make electricity which was discharged on gold electrodes in a Leyden jar with water.[1] In 1800 Alessandro Volta invented the voltaic pile, and a few weeks later the English scientists William Nicholson and Anthony Carlisle used it for the electrolysis of water. In 1806 Humphry Davy reported the results of extensive distilled water electrolysis experiments concluding that nitric acid was produced at the anode from dissolved atmospheric nitrogen gas. He used a high voltage battery and non-reactive electrodes and vessels such as gold electrode cones that doubled as vessels bridged by damp asbestos.[2] When Zénobe Gramme invented the Gramme machine in 1869 electrolysis of water became a cheap method for the production of hydrogen. A method of industrial synthesis of hydrogen and oxygen through electrolysis was developed by Dmitry Lachinov in 1888.[3]
Principle[]
A DC electrical power source is connected to two electrodes, or two plates (typically made from an inert metal such as platinum or iridium) which are placed in the water. Hydrogen will appear at the cathode (where electrons enter the water), and oxygen will appear at the anode.[4] Assuming ideal faradaic efficiency, the amount of hydrogen generated is twice the amount of oxygen, and both are proportional to the total electrical charge conducted by the solution.[5] However, in many cells competing side reactions occur, resulting in different products and less than ideal faradaic efficiency.
Electrolysis of pure water requires excess energy in the form of overpotential to overcome various activation barriers. Without the excess energy, the electrolysis of pure water occurs very slowly or not at all. This is in part due to the limited self-ionization of water. Pure water has an electrical conductivity about one-millionth that of seawater. Many electrolytic cells may also lack the requisite electrocatalysts. The efficiency of electrolysis is increased through the addition of an electrolyte (such as a salt, an acid or a base) and the use of electrocatalysts.
Currently the electrolytic process is rarely used in industrial applications since hydrogen can currently be produced more affordably from fossil fuels.[6]
Equations[]

In pure water at the negatively charged cathode, a reduction reaction takes place, with electrons (e−) from the cathode being given to hydrogen cations to form hydrogen gas. The half reaction, balanced with acid, is:
- Reduction at cathode: 2 H+(aq) + 2e− → H2(g)
At the positively charged anode, an oxidation reaction occurs, generating oxygen gas and giving electrons to the anode to complete the circuit:
- Oxidation at anode: 2 H2O(l) → O2(g) + 4 H+(aq) + 4e−
The same half-reactions can also be balanced with the base as listed below. Not all half-reactions must be balanced with acid or base. Many do, like the oxidation or reduction of water listed here. To add half reactions they must both be balanced with either acid or base. The acid-balanced reactions predominate in acidic (low pH) solutions, while the base-balanced reactions predominate in basic (high pH) solutions.
| Cathode (reduction): | 2 H2O(l) + 2e− | → | H2(g) + 2 OH−(aq) |
| Anode (oxidation): | 2 OH−(aq) | → | 1/2 O2(g) + H2O(l) + 2 e− |
Combining either half reaction pair yields the same overall decomposition of water into oxygen and hydrogen:
- Overall reaction: 2 H2O(l) → 2 H2(g) + O2(g)
The number of hydrogen molecules produced is thus twice the number of oxygen molecules. Assuming equal temperature and pressure for both gases, the produced hydrogen gas has, therefore, twice the volume of the produced oxygen gas. The number of electrons pushed through the water is twice the number of generated hydrogen molecules and four times the number of generated oxygen molecules.
Thermodynamics[]
The decomposition of pure water into hydrogen and oxygen at standard temperature and pressure is not favorable in thermodynamic terms.
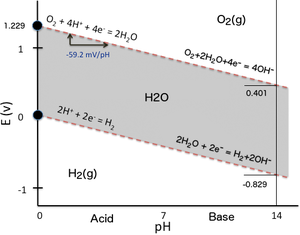
| Anode (oxidation): | 2 H2O(l) | → | O2(g) + 4 H+(aq) + 4e− | Eo = +1.23 V (for the reduction half-equation)[7] | |
| Cathode (reduction): | 2 H+(aq) + 2e− | → | H2(g) | Eo = 0.00 V |
Thus, the standard potential of the water electrolysis cell (Eocell = Eocathode − Eoanode) is -1.229 V at 25 °C at pH 0 ([H+] = 1.0 M). At 25 °C with pH 7 ([H+] = 1.0×10−7 M), the potential is unchanged based on the Nernst equation. The thermodynamic standard cell potential can be obtained from standard-state free energy calculations to find ΔG° and then using the equation: ΔG°= −n F E° (where E° is the cell potential and F the Faraday constant, i.e. 96,485.3321233 C/mol). For two water molecules electrolysed and hence two hydrogen molecules formed, n = 4, and ΔG° = 474.48 kJ/2 mol(water) = 237.24 kJ/mol(water), and ΔS° = 163 J/K mol(water), and ΔH° = 571.66 kJ/2 mol(water) = 285.83 kJ/mol(water), and finally 141.86 MJ/kg(H2) . However, calculations regarding individual electrode equilibrium potentials requires some corrections taking into account the activity coefficients.[8] In practice when an electrochemical cell is "driven" toward completion by applying reasonable potential, it is kinetically controlled. Therefore, activation energy, ion mobility (diffusion) and concentration, wire resistance, surface hindrance including bubble formation (causes electrode area blockage), and entropy, require a greater applied potential to overcome these factors. The amount of increase in potential required is termed the overpotential.
Electrolyte selection[]

If the above described processes occur in pure water, H+ cations will be consumed/reduced at the cathode and OH− anions will be consumed/oxidised at the anode. This can be verified by adding a pH indicator to the water: the water near the cathode is basic while the water near the anode is acidic. The negative hydroxide ions that approach the anode mostly combine with the positive hydronium ions (H3O+) to form water. The positive hydronium ions that approach the cathode mostly combine with negative hydroxide ions to form water. Relatively few hydroniums/hydroxide ions reach the cathode/anode. This can cause a concentration overpotential at both electrodes.
Pure water is a fairly good insulator since it has a low autoionization, Kw = 1.0×10−14 at room temperature and thus pure water conducts current poorly, 0.055 µS·cm−1.[9] Unless a very large potential is applied to cause an increase in the autoionization of water the electrolysis of pure water proceeds very slowly limited by the overall conductivity.
If a water-soluble electrolyte is added, the conductivity of the water rises considerably. The electrolyte disassociates into cations and anions; the anions rush towards the anode and neutralize the buildup of positively charged H+ there; similarly, the cations rush towards the cathode and neutralize the buildup of negatively charged OH− there. This allows the continuous flow of electricity.[10]
Electrolyte for water electrolysis[]
Care must be taken in choosing an electrolyte since an anion from the electrolyte competes with the hydroxide ions to give up an electron. An electrolyte anion with less standard electrode potential than hydroxide will be oxidized instead of the hydroxide, and no oxygen gas will be produced. A cation with a greater standard electrode potential than a hydrogen ion will be reduced instead, and no hydrogen gas will be produced.
The following cations have lower electrode potential than H+ and are therefore suitable for use as electrolyte cations: Li+, Rb+, K+, Cs+, Ba2+, Sr2+, Ca2+, Na+, and Mg2+. Sodium and lithium are frequently used, as they form inexpensive, soluble salts.
If an acid is used as the electrolyte, the cation is H+, and there is no competitor for the H+ created by disassociating water. The most commonly used anion is sulfate (SO2−
4), as it is very difficult to oxidize, with the standard potential for oxidation of this ion to the peroxydisulfate ion being +2.010 volts.[11]
Strong acids such as sulfuric acid (H2SO4), and strong bases such as potassium hydroxide (KOH), and sodium hydroxide (NaOH) are frequently used as electrolytes due to their strong conducting abilities.
A solid polymer electrolyte can also be used such as Nafion and when applied with a special catalyst on each side of the membrane can efficiently split the water molecule with as little as 1.5 volts. Several other solid electrolyte systems have been trialed and developed with several electrolysis systems now available commercially that use solid electrolytes.[12]
Pure water electrolysis[]
Electrolyte-free pure water electrolysis has been achieved by using deep-sub-Debye-length nanogap electrochemical cells. When the gap distance between cathode and anode even smaller than Debye-length (1 micron in pure water, around 220 nm in distilled water), the double layer regions from two electrodes can overlap, leading to uniformly high electric field distributed inside the entire gap. Such a high electric field can significantly enhance the ion transport inside the water (mainly due to migration), further enhancing self-ionization of water and keeping the whole reaction continuing, and showing small resistance between the two electrodes. In this case, the two half-reactions are coupled together and limited by electron-transfer steps (electrolysis current saturated when further reducing the electrode distance).[13]
Techniques[]
Fundamental demonstration[]
Two leads, running from the terminals of a battery, are placed in a cup of water with a quantity of electrolyte to establish conductivity in the solution. Using NaCl (table salt) in an electrolyte solution results in chlorine gas rather than oxygen due to a competing half-reaction. With the correct electrodes and correct electrolyte, such as baking soda (sodium bicarbonate), hydrogen and oxygen gases will stream from the oppositely charged electrodes. Oxygen will collect at the positively charged electrode (anode) and hydrogen will collect at the negatively charged electrode (cathode). Note that hydrogen is positively charged in the H2O molecule, so it ends up at the negative electrode. (And vice versa for oxygen.)
Note that an aqueous solution of water with chloride ions, when electrolyzed, will result in either OH− if the concentration of Cl− is low, or in chlorine gas being preferentially discharged if the concentration of Cl− is greater than 25% by mass in the solution.

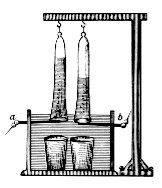
Hofmann voltameter[]
The Hofmann voltameter is often used as a small-scale electrolytic cell. It consists of three joined upright cylinders. The inner cylinder is open at the top to allow the addition of water and the electrolyte. A platinum electrode is placed at the bottom of each of the two side cylinders, connected to the positive and negative terminals of a source of electricity. When current is run through the Hofmann voltameter, gaseous oxygen forms at the anode (positive) and gaseous hydrogen at the cathode (negative). Each gas displaces water and collects at the top of the two outer tubes, where it can be drawn off with a stopcock.
Industrial[]
Many industrial electrolysis cells are very similar to Hofmann voltameters, with complex platinum plates or honeycombs as electrodes. Generally, the only time hydrogen is intentionally produced from electrolysis is for the specific point of use application such as is the case with oxyhydrogen torches or when extremely high purity hydrogen or oxygen is desired. The vast majority of hydrogen is produced from hydrocarbons and as a result, contains trace amounts of carbon monoxide among other impurities. The carbon monoxide impurity can be detrimental to various systems including many fuel cells.
High-pressure[]
High-pressure electrolysis is the electrolysis of water with a compressed hydrogen output around 12–20 MPa (120–200 Bar, 1740–2900 psi).[14] By pressurising the hydrogen in the electrolyser, the need for an external hydrogen compressor is eliminated; the average energy consumption for internal compression is around 3%.[15]
High-temperature[]
High-temperature electrolysis (also HTE or steam electrolysis) is a method currently being investigated for water electrolysis with a heat engine. High temperature electrolysis may be preferable to traditional room-temperature electrolysis because some of the energy is supplied as heat, which is cheaper than electricity, and because the electrolysis reaction is more efficient at higher temperatures.[16][17]
Alkaline water[]
Polymer electrolyte membrane[]
Nickel/iron[]
In 2014, researchers announced an electrolysis system made of inexpensive, abundant nickel and iron rather than precious metal catalysts, such as platinum or iridium. The nickel-metal/nickel-oxide structure is more active than pure nickel metal or pure nickel oxide alone. The catalyst significantly lowers the required voltage.[18][19] Also nickel–iron batteries are being investigated for use as combined batteries and electrolysis for hydrogen production. Those "battolysers" could be charged and discharged like conventional batteries, and would produce hydrogen when fully charged.[20]
Nanogap electrochemical cells[]
In 2017, researchers reported using nanogap electrochemical cells to achieve high-efficiency electrolyte-free pure water electrolysis at room temperature. In nanogap electrochemical cells, the two electrodes are so close to each other (even smaller than Debye-length in pure water) that the mass transport rate can be even higher than the electron-transfer rate, leading to two half-reactions coupled together and limited by electron-transfer step. Experiments show that the electrical current density from pure water electrolysis can be even larger than that from 1 mol/L sodium hydroxide solution. The mechanism, "Virtual Breakdown Mechanism", is completely different from the well-established traditional electrochemical theory, due to such nanogap size effect.[13]
Applications[]
About five percent of hydrogen gas produced worldwide is created by electrolysis. Currently, most industrial methods produce hydrogen from natural gas instead, in the steam reforming process. The majority of the hydrogen produced through electrolysis is a side product in the production of chlorine and caustic soda. This is a prime example of a competing for side reaction.
- 2NaCl + 2H2O → Cl2 + H2 + 2NaOH
In the chloralkali process (electrolysis of brine) a water/sodium chloride mixture is only half the electrolysis of water since the chloride ions are oxidized to chlorine rather than water being oxidized to oxygen. Thermodynamically, this would not be expected since the oxidation potential of the chloride ion is less than that of water, but the rate of the chloride reaction is much greater than that of water, causing it to predominate. The hydrogen produced from this process is either burned (converting it back to water), used for the production of specialty chemicals, or various other small-scale applications.
Water electrolysis is also used to generate oxygen for the International Space Station.[21][22]
Hydrogen may later be used in a fuel cell as a storage method of energy and water.[23]
Additionally, many car companies have recently begun researching using water as a fuel source, converting it into hydrogen and oxygen via water electrolysis, and using the hydrogen as fuel in a Hydrogen vehicle, however, have not met much success due to the unstable characteristics of hydrogen as a fuel source.
Efficiency[]
Industrial output[]
Efficiency of modern hydrogen generators is measured by energy consumed per standard volume of hydrogen (MJ/m3), assuming standard temperature and pressure of the H2. The lower the energy used by a generator, the higher its efficiency would be; a 100%-efficient electrolyser would consume 39.4 kilowatt-hours per kilogram (142 MJ/kg) of hydrogen,[24] 12,749 joules per litre (12.75 MJ/m3). Practical electrolysis (using a rotating electrolyser at 15 bar pressure) may consume 50 kW⋅h/kg (180 MJ/kg), and a further 15 kW⋅h (54 MJ) if the hydrogen is compressed for use in hydrogen cars.[25]
Electrolyzer vendors provide efficiencies based on enthalpy. To assess the claimed efficiency of an electrolyzer it is important to establish how it was defined by the vendor (i.e. what enthalpy value, what current density, etc.).
There are two main technologies available on the market, alkaline and proton exchange membrane (PEM) electrolyzers. Alkaline electrolyzers are cheaper in terms of investment (they generally use nickel catalysts), but less efficient; PEM electrolyzers, conversely, are more expensive (they generally use expensive platinum-group metal catalysts) but are more efficient and can operate at higher current densities, and can, therefore, be possibly cheaper if the hydrogen production is large enough.
Conventional alkaline electrolysis has an efficiency of about 70%.[26] Accounting for the accepted use of the higher heat value (because inefficiency via heat can be redirected back into the system to create the steam required by the catalyst), average working efficiencies for PEM electrolysis are around 80%.[27][28] This is expected to increase to between 82–86%[29] before 2030. Theoretical efficiency for PEM electrolysers are predicted up to 94%.[30]

Considering the industrial production of hydrogen, and using current best processes for water electrolysis (PEM or alkaline electrolysis) which have an effective electrical efficiency of 70–80%,[31][32][33] producing 1 kg of hydrogen (which has a specific energy of 143 MJ/kg) requires 50–55 kW⋅h (180–200 MJ) of electricity. At an electricity cost of $0.06/kW·h, as set out in the US Department of Energy hydrogen production targets for 2015,[34] the hydrogen cost is $3/kg. With the range of natural gas prices from 2016 as shown in the graph (Hydrogen Production Tech Team Roadmap, November 2017) putting the cost of steam-methane-reformed (SMR) hydrogen at between $1.20 and $1.50, the cost price of hydrogen via electrolysis is still over double 2015 DOE hydrogen target prices. The US DOE target price for hydrogen in 2020 is $2.30/kg, requiring an electricity cost of $0.037/kW·h, which is achievable given 2018 PPA tenders[35] for wind and solar in many regions. This puts the $4/gasoline gallon equivalent (gge) H2 dispensed objective well within reach, and close to a slightly elevated natural gas production cost for SMR.
In other parts of the world, the price of SMR hydrogen is between $1–3/kg on average. This makes production of hydrogen via electrolysis cost competitive in many regions already, as outlined by Nel Hydrogen[36] and others, including an article by the IEA[37] examining the conditions which could lead to a competitive advantage for electrolysis.
Overpotential[]
Real water electrolyzers require higher voltages for the reaction to proceed. The part that exceeds 1.23 V[38] is called overpotential or overvoltage, and represents any kind of loss and nonideality in the electrochemical process.
For a well designed cell the largest overpotential is the reaction overpotential for the four-electron oxidation of water to oxygen at the anode; electrocatalysts can facilitate this reaction, and platinum alloys are the state of the art for this oxidation. Developing a cheap, effective electrocatalyst for this reaction would be a great advance, and is a topic of current research; there are many approaches, among them a 30-year-old recipe for molybdenum sulfide,[39] graphene quantum dots,[40] carbon nanotubes,[19] perovskite,[41] and nickel/nickel-oxide.[42][43] Tri‐molybdenum phosphide (Mo3P) has been recently found as a promising nonprecious metal and earth‐abundant candidate with outstanding catalytic properties that can be used for electrocatalytic processes. The catalytic performance of Mo3P nanoparticles is tested in the hydrogen evolution reaction (HER), indicating an onset potential of as low as 21 mV, H2 formation rate, and exchange current density of 214.7 µmol s−1 g−1 cat (at only 100 mV overpotential) and 279.07 µA cm−2, respectively, which are among the closest values yet observed to platinum.[44][45] The simpler two-electron reaction to produce hydrogen at the cathode can be electrocatalyzed with almost no overpotential by platinum, or in theory a hydrogenase enzyme. If other, less effective, materials are used for the cathode (e.g. graphite), large overpotentials will appear.
Thermodynamics[]
The electrolysis of water in standard conditions requires a theoretical minimum of 237 kJ of electrical energy input to dissociate each mole of water, which is the standard Gibbs free energy of formation of water. It also requires energy to overcome the change in entropy of the reaction. Therefore, the process cannot proceed below 286 kJ per mol if no external heat/energy is added.
Since each mole of water requires two moles of electrons, and given that the Faraday constant F represents the charge of a mole of electrons (96485 C/mol), it follows that the minimum voltage necessary for electrolysis is about 1.23 V.[46] If electrolysis is carried out at high temperature, this voltage reduces. This effectively allows the electrolyser to operate at more than 100% electrical efficiency. In electrochemical systems this means that heat must be supplied to the reactor to sustain the reaction. In this way thermal energy can be used for part of the electrolysis energy requirement.[47] In a similar way the required voltage can be reduced (below 1 V) if fuels (such as carbon, alcohol, biomass) are reacted with water (PEM based electrolyzer in low temperature) or oxygen ions (solid oxide electrolyte based electrolyzer in high temperature). This results in some of the fuel's energy being used to "assist" the electrolysis process and can reduce the overall cost of hydrogen produced.[48]
However, observing the entropy component (and other losses), voltages over 1.48 V are required for the reaction to proceed at practical current densities (the thermoneutral voltage).
In the case of water electrolysis, Gibbs free energy represents the minimum work necessary for the reaction to proceed, and the reaction enthalpy is the amount of energy (both work and heat) that has to be provided so the reaction products are at the same temperature as the reactant (i.e. standard temperature for the values given above). Potentially, an electrolyzer operating at 1.48 V would be 100% efficient.[citation needed]
See also[]
References[]
- ^ Levie, R. de (October 1999). "The electrolysis of water". Journal of Electroanalytical Chemistry. 476 (1): 92–93. doi:10.1016/S0022-0728(99)00365-4.[dead link]
- ^ Davy, John, ed. (1839). "On Some Chemical Agencies of Electricity". The Collected Works of Sir Humphry Davy. 5. pp. 1–12.
- ^ Lachinov Dmitry Aleksandrovich Archived 26 July 2011 at the Wayback Machine at Great Cyrill and Methodius Encyclopedia (in Russian)
- ^ Zumdahl, Steven S.; Zumdahl, Susan A. (1 January 2013). Chemistry (9th ed.). Cengage Learning. p. 866. ISBN 978-1-13-361109-7.
- ^ Carmo, M; Fritz D; Mergel J; Stolten D (2013). "A comprehensive review on PEM water electrolysis". Journal of Hydrogen Energy. 38 (12): 4901–4934. doi:10.1016/j.ijhydene.2013.01.151.
- ^ "Hydrogen Basics — Production". Florida Solar Energy Center. 2007. Archived from the original on 18 February 2008. Retrieved 5 February 2008.
- ^ standard electrode potential (data page)
- ^ Colli, A.N.; et al. (2019). "Non-Precious Electrodes for Practical Alkaline Water Electrolysis". Materials. 12 (8): 1336. doi:10.3390/ma12081336. PMC 6515460. PMID 31022944.
- ^ Light, Truman S.; Licht, Stuart; Bevilacqua, Anthony C.; Morash, Kenneth R. (1 January 2005). "The Fundamental Conductivity and Resistivity of Water". Electrochemical and Solid-State Letters. 8 (1): E16–E19. doi:10.1149/1.1836121. ISSN 1099-0062. S2CID 54511887.
- ^ Pauling, Linus (1970) General Chemistry, Section 15-2. San Francisco.
- ^ CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data. Haynes, William M. (93rd ed., 2012-2013 ed.). Boca Raton, Fla.: CRC. 2012. ISBN 9781439880494. OCLC 793213751.CS1 maint: others (link)
- ^ Badwal, SPS; Giddey S; Munnings C (2012). "Hydrogen production via solid electrolytic routes". WIREs Energy and Environment. 2 (5): 473–487. doi:10.1002/wene.50. Archived from the original on 2 June 2013. Retrieved 23 January 2013.
- ^ Jump up to: a b Wang, Yifei; Narayanan, S. R.; Wu, Wei (11 July 2017). "Field-Assisted Splitting of Pure Water Based on Deep-Sub-Debye-Length Nanogap Electrochemical Cells". ACS Nano. 11 (8): 8421–8428. doi:10.1021/acsnano.7b04038. ISSN 1936-0851. PMID 28686412.
- ^ 2001-High pressure electrolysis – The key technology for efficient H.2[dead link]
- ^ Ghosh, P.C; Emonts, B; Janßen, H; Mergel, J; Stolten, D (2003). "Ten years of operational experience with a hydrogen-based renewable energy supply system" (PDF). Solar Energy. 75 (6): 469–478. Bibcode:2003SoEn...75..469G. doi:10.1016/j.solener.2003.09.006. Archived from the original on 27 March 2009.CS1 maint: unfit URL (link)
- ^ "High temperature electrolysis using SOEC". Hi2h2. Archived from the original on 3 March 2016. Retrieved 5 May 2016.
- ^ "WELTEMPWater electrolysis at elevated temperatures". Weltemp.eu. 31 December 2010. Archived from the original on 3 March 2016. Retrieved 5 May 2016.
- ^ "A low-cost water splitter that runs on an ordinary AAA battery". KurzweilAI. 22 August 2014. Archived from the original on 16 April 2015. Retrieved 11 April 2015.
- ^ Jump up to: a b Gong, Ming; Zhou, Wu; Tsai, Mon-Che; Zhou, Jigang; Guan, Mingyun; Lin, Meng-Chang; Zhang, Bo; Hu, Yongfeng; Wang, Di-Yan; Yang, Jiang; Pennycook, Stephen J.; Hwang, Bing-Joe; Dai, Hongjie (2014). "Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis". Nature Communications. 5: 4695. Bibcode:2014NatCo...5.4695G. doi:10.1038/ncomms5695. PMID 25146255.
- ^ Mulder, F. M.; et al. (2017). "Efficient electricity storage with the battolyser, an integrated Ni-Fe-battery and electrolyser". Energy and Environmental Science. 10 (3): 756–764. doi:10.1039/C6EE02923J.
- ^ "Making Space Safer with Electrolysis". ASME. Archived from the original on 15 May 2012. Retrieved 26 May 2012.
- ^ "Breathing Easy on the Space Station". NASA Science. Archived from the original on 19 May 2012. Retrieved 26 May 2012.
- ^ "Solar Hydrogen Fuel Cell Water Heater (Educational Stand)". Archived from the original on 8 August 2016. Retrieved 9 September 2017 – via Scribd.
- ^ Luca Bertuccioli; et al. (7 February 2014). "Development of water electrolysis in the European Union" (PDF). Client Fuel Cells and Hydrogen Joint Undertaking.
- ^ Stensvold, Tore (26 January 2016). «Coca-Cola-oppskrift» kan gjøre hydrogen til nytt norsk industrieventyr Archived 5 March 2016 at the Wayback Machine. Teknisk Ukeblad, .
- ^ Stolten, Detlef (4 January 2016). Hydrogen Science and Engineering: Materials, Processes, Systems and Technology. John Wiley & Sons. p. 898. ISBN 9783527674299. Archived from the original on 22 April 2018. Retrieved 22 April 2018.
- ^ Bernholz, Jan (13 September 2018). "RWE's former, current and possible future energy storage applications" (PDF). RWE. p. 10.
Total Efficiency: 70%, or 86% (usage of waste heat)
- ^ "ITM – Hydrogen Refuelling Infrastructure – February 2017" (PDF). level-network.com. Archived (PDF) from the original on 17 April 2018. Retrieved 17 April 2018.
- ^ "Cost reduction and performance increase of PEM electrolysers" (PDF). Europa (web portal). Archived (PDF) from the original on 17 April 2018. Retrieved 17 April 2018.
- ^ Bjørnar Kruse; Sondre Grinna; Cato Buch (13 February 2002). "Hydrogen—Status and Possibilities" (PDF). The Bellona Foundation. p. 20. Archived from the original on 16 September 2013.CS1 maint: unfit URL (link)
- ^ Werner Zittel; Reinhold Wurster (8 July 1996). "Chapter 3: Production of Hydrogen. Part 4: Production from electricity by means of electrolysis". HyWeb: Knowledge – Hydrogen in the Energy Sector. Ludwig-Bölkow-Systemtechnik GmbH. Archived from the original on 7 February 2007. Retrieved 14 January 2006.
- ^ Bjørnar Kruse; Sondre Grinna; Cato Buch (13 February 2002). "Hydrogen—Status and Possibilities". The Bellona Foundation. Archived from the original (PDF) on 2 July 2011.
Efficiency factors for PEM electrolysers up to 94% are predicted, but this is only theoretical at this time.
- ^ "high-rate and high efficiency 3D water electrolysis". Grid-shift.com. Archived from the original on 22 March 2012. Retrieved 13 December 2011.
- ^ "DOE Technical Targets for Hydrogen Production from Electrolysis". energy.gov. US Department of Energy. Archived from the original on 23 April 2018. Retrieved 22 April 2018.
- ^ Deign, Jason. "Xcel Attracts 'Unprecedented' Low Prices for Solar and Wind Paired With Storage". greentechmedia.com. Wood MacKenzie. Archived from the original on 4 February 2018. Retrieved 22 April 2018.
- ^ "Wide Spread Adaption of Competitive Hydrogen Solution" (PDF). nelhydrogen.com. Nel ASA. Archived (PDF) from the original on 22 April 2018. Retrieved 22 April 2018.
- ^ Philibert, Cédric. "Commentary: Producing industrial hydrogen from renewable energy". iea.org. International Energy Agency. Archived from the original on 22 April 2018. Retrieved 22 April 2018.
- ^ 1.23 V is the standard potential; in non-standard conditions it may be different, in particular, it decreases with temperature.
- ^ Kibsgaard, Jakob; Jaramillo, Thomas F.; Besenbacher, Flemming (2014). "Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2− clusters". Nature Chemistry. 6 (3): 248–253. Bibcode:2014NatCh...6..248K. doi:10.1038/nchem.1853. PMID 24557141.
- ^ Fei, Huilong; Ye, Ruquan; Ye, Gonglan; Gong, Yongji; Peng, Zhiwei; Fan, Xiujun; Samuel, Errol L. G.; Ajayan, Pulickel M.; Tour, James M. (2014). "Boron- and Nitrogen-Doped Graphene Quantum Dots/Graphene Hybrid Nanoplatelets as Efficient Electrocatalysts for Oxygen Reduction". ACS Nano. 8 (10): 10837–43. doi:10.1021/nn504637y. PMID 25251218.
- ^ Luo, J.; Im, J.-H.; Mayer, M. T.; Schreier, M.; Nazeeruddin, M. K.; Park, N.-G.; Tilley, S. D.; Fan, H. J.; Gratzel, M. (2014). "Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts". Science. 345 (6204): 1593–1596. Bibcode:2014Sci...345.1593L. doi:10.1126/science.1258307. PMID 25258076.
- ^ Shwartz, Mark (22 August 2014). "Stanford scientists develop water splitter that runs on ordinary AAA battery". News.stanford.edu. Archived from the original on 16 April 2016. Retrieved 5 May 2016.
- ^ "Scientists develop a water splitter that runs on an ordinary AAA battery". Technology.org. 25 August 2014. Retrieved 5 May 2016.
- ^ Kondori, Alireza (2 May 2019). "Identifying Catalytic Active Sites of Trimolybdenum Phosphide (Mo3P) for Electrochemical Hydrogen Evolution". Advanced Energy Materials. AdvancedEnergyMaterials. 9 (22): 1900516. doi:10.1002/aenm.201900516.
- ^ Shi, Yanmei (25 January 2016). "Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction". Chemical Society Reviews. ChemicalSocietyReviews. 45 (6): 1529–1541. doi:10.1039/C5CS00434A. PMID 26806563.
- ^ Hyman D. Gesser (2002). Applied Chemistry. Springer. pp. 16–. ISBN 978-0-306-46700-4. Retrieved 18 December 2011.
- ^ Badwal, Sukhvinder P.S.; Giddey, Sarbjit; Munnings, Christopher (September 2013). "Hydrogen production via solid electrolytic routes". Wiley Interdisciplinary Reviews: Energy and Environment. 2 (5): 473–487. doi:10.1002/wene.50. S2CID 135539661.
- ^ Badwal, Sukhvinder P. S.; Giddey, Sarbjit S.; Munnings, Christopher; Bhatt, Anand I.; Hollenkamp, Anthony F. (24 September 2014). "Emerging electrochemical energy conversion and storage technologies (open access)". Frontiers in Chemistry. 2: 79. Bibcode:2014FrCh....2...79B. doi:10.3389/fchem.2014.00079. PMC 4174133. PMID 25309898.
External links[]
| Wikimedia Commons has media related to Water electrolysis. |
- "Electrolysis of Water". Experiments on Electrochemistry. Retrieved 20 November 2005.
- "Electrolysis of Water". Do Chem 044. Archived from the original on 14 March 2006. Retrieved 20 November 2005.
- EERE 2008 – 100 kgH2/day Trade Study
- NREL 2006 – Electrolysis technical report
- Electrolysis
- Hydrogen production
- Industrial gases
- Water chemistry