Group (periodic table)
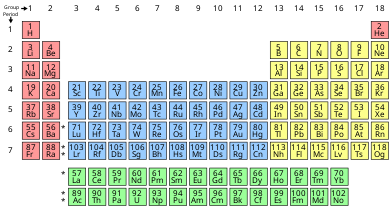
In chemistry, a group (also known as a family[1]) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the f-block columns (between groups 2 and 3) are not numbered. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms (i.e., the same core charge), because most chemical properties are dominated by the orbital location of the outermost electron.
There are three systems of group numbering for the groups; the same number may be assigned to different groups depending on the system being used. The modern numbering system of "group 1" to "group 18" has been recommended by the International Union of Pure and Applied Chemistry (IUPAC) since about 1990. It replaces two older incompatible naming schemes, used by the Chemical Abstract Service (CAS, more popular in the US), and by IUPAC before 1990 (more popular in Europe). The system of eighteen groups is generally accepted by the chemistry community, but some dissent exists about membership of several elements. Disagreements mostly involve elements number 1 and 2 (hydrogen and helium), as well as inner transition metals.
Groups may also be identified using their topmost element, or have a specific name. For example, group 16 is also described as the "oxygen group" and as the "chalcogens". An exception is the "iron group", which usually refers to "group 8", but in chemistry may also mean iron, cobalt, and nickel, or some other set of elements with similar chemical properties. In astrophysics and nuclear physics, it usually refers to iron, cobalt, nickel, chromium, and manganese.
Group names[]
In history, several sets of group names have been used:[2][3]
| IUPAC group | 1a | 2 | n/a | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mendeleev (I–VIII) | I | II | III | IV | V | VI | VII | VIII | I | II | III | IV | V | VI | VII | b | |||
| CAS (US, A-B-A) | IA | IIA | IIIB | IVB | VB | VIB | VIIB | VIIIB | IB | IIB | IIIA | IVA | VA | VIA | VIIA | VIIIA | |||
| old IUPAC (Europe, A-B) | IA | IIA | IIIA | IVA | VA | VIA | VIIA | VIII | IB | IIB | IIIB | IVB | VB | VIB | VIIB | 0 | |||
| Trivial name | H and Alkali metalsr | Alkaline earth metalsr | Coinage metals | Triels | Tetrels | Pnictogensr | Chalcogensr | Halogensr | Noble gasesr | ||||||||||
| Name by elementr | Lithium group | Beryllium group | Scandium group | Titanium group | Vanadium group | Chromium group | Manganese group | Iron group | Cobalt group | Nickel group | Copper group | Zinc group | Boron group | Carbon group | Nitrogen group | Oxygen group | Fluorine group | Helium or Neon group | |
| Period 1 | H | He | |||||||||||||||||
| Period 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||
| Period 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| Period 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Period 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Period 6 | Cs | Ba | La–Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Period 7 | Fr | Ra | Ac–No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
n/a Do not have a group number
b Group 18, the noble gases, were not discovered at the time of Mendeleev's original table. Later (1902), Mendeleev accepted the evidence for their existence, and they could be placed in a new "group 0", consistently and without breaking the periodic table principle.
r Group name as recommended by IUPAC.
| New IUPAC name |
Old IUPAC (Europe) |
CAS name (U.S.) |
Name by element |
IUPAC recommended trivial name |
Other trivial name |
|---|---|---|---|---|---|
| Group 1 | IA | IA | lithium family |
hydrogen and alkali metals* |
|
| Group 2 | IIA | IIA | beryllium family | alkaline earth metals* | |
| Group 3 | IIIA | IIIB | scandium family | ||
| Group 4 | IVA | IVB | titanium family | ||
| Group 5 | VA | VB | vanadium family | ||
| Group 6 | VIA | VIB | chromium family | ||
| Group 7 | VIIA | VIIB | manganese family | ||
| Group 8 | VIII | VIIIB | iron family | ||
| Group 9 | VIII | VIIIB | cobalt family | ||
| Group 10 | VIII | VIIIB | nickel family | ||
| Group 11 | IB | IB | copper family | coinage metals | |
| Group 12 | IIB | IIB | zinc family | ||
| Group 13 | IIIB | IIIA | boron family | triels from Greek tri (three, III)[4][5] | |
| Group 14 | IVB | IVA | carbon family | tetrels from Greek tetra (four, IV)[4][5] | |
| Group 15 | VB | VA | nitrogen family | pnictogens* | pentels from Greek penta (five, V)[5] |
| Group 16 | VIB | VIA | oxygen family | chalcogens* | |
| Group 17 | VIIB | VIIA | fluorine family | halogens* | |
| Group 18 | 0 | VIIIA | helium family or neon family |
noble gases* |
Some other names have been proposed and used without gaining wide acceptance: "volatile metals" for group 12;[6] "icosagens" for group 13;[7] "crystallogens",[4] "adamantogens",[8] and "merylides"[citation needed] for group 14; and "aerogens" for group 18.[5]
CAS and old IUPAC numbering (A/B)[]
Two earlier group number systems exist: CAS (Chemical Abstracts Service) and old IUPAC. Both use numerals (Arabic or Roman) and letters A and B. Both systems agree on the numbers. The numbers indicate approximately the highest oxidation number of the elements in that group, and so indicate similar chemistry with other elements with the same numeral. The number proceeds in a linearly increasing fashion for the most part, once on the left of the table, and once on the right (see List of oxidation states of the elements), with some irregularities in the transition metals. However, the two systems use the letters differently. For example, potassium (K) has one valence electron. Therefore, it is located in group 1. Calcium (Ca) is in group 2, for it contains two valence electrons.
In the old IUPAC system the letters A and B were designated to the left (A) and right (B) part of the table, while in the CAS system the letters A and B are designated to main group elements (A) and transition elements (B). The old IUPAC system was frequently used in Europe, while the CAS is most common in America. The new IUPAC scheme was developed to replace both systems as they confusingly used the same names to mean different things. The new system simply numbers the groups increasingly from left to right on the standard periodic table. The IUPAC proposal was first circulated in 1985 for public comments,[2] and was later included as part of the 1990 edition of the Nomenclature of Inorganic Chemistry.[9]
See also[]
References[]
- ^ "The Periodic Table Terms". www.shmoop.com. Retrieved 2018-09-15.
- ^ Jump up to: a b Fluck, E. (1988). "New Notations in the Periodic Table" (PDF). Pure Appl. Chem. IUPAC. 60 (3): 431–436. doi:10.1351/pac198860030431. S2CID 96704008. Retrieved 24 March 2012.
- ^ IUPAC (2005). "Nomenclature of inorganic chemistry" (PDF).
- ^ Jump up to: a b c Liu, Ning; Lu, Na; Su, Yan; Wang, Pu; Quan, Xie (2019). "Fabrication of g-C3N4/Ti3C2 composite and its visible-light photocatalytic capability for ciprofloxacin degradation". Separation and Purification Technology. 211: 782–789. doi:10.1016/j.seppur.2018.10.027. Retrieved 17 August 2019.
- ^ Jump up to: a b c d Rich, Ronald (2007). Inorganic Reactions in Water. Springer. pp. 307, 327, 363, 475. doi:10.1007/978-3-540-73962-3. ISBN 9783540739616.
- ^ https://glosbe.com/en/en/volatile%20metal
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 227. ISBN 978-0-08-037941-8.
- ^ W. B. Jensen, The Periodic Law and Table
- ^ Leigh, G. J. Nomenclature of Inorganic Chemistry: Recommendations 1990. Blackwell Science, 1990. ISBN 0-632-02494-1.
Further reading[]
- Scerri, E. R. (2007). The periodic table, its story and its significance. Oxford University Press. ISBN 978-0-19-530573-9.
- Periodic table
- Groups (periodic table)