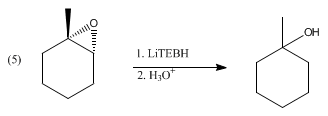Lithium triethylborohydride

| |
| Names | |
|---|---|
| Preferred IUPAC name
Lithium triethylboranuide | |
| Other names
Superhydride
LiTEBH | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.040.963 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Li(C2H5)3BH | |
| Molar mass | 105.95 g/mol |
| Appearance | Colorless to yellow liquid |
| Density | 0.890 g/cm3, liquid |
| Boiling point | 66 °C (151 °F; 339 K) for THF |
| reactive | |
| Hazards | |
| Main hazards | highly flammable corrosive Causes burns Probable Carcinogen |
| Safety data sheet (SDS) | External MSDS |
| GHS labelling:[1] | |
  
| |
Signal word
|
Danger |
| H250, H260, H314, H335 | |
| P210, P222, P223, P231+P232, P260, P261, P264, P271, P280, P301+P330+P331, P302+P334, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P335+P334, P363, P370+P378, P402+P404, P403+P233, P405, P422, P501 | |
| NFPA 704 (fire diamond) | 
3
2
2 |
| Related compounds | |
Related hydride
|
Lithium borohydride sodium borohydride sodium hydride lithium aluminium hydride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lithium triethylborohydride is the organoboron compound with the formula LiEt3BH. Commonly referred to as LiTEBH or Superhydride, it is a powerful reducing agent used in organometallic and organic chemistry. It is a colorless or white liquid but is typically marketed and used as a THF solution.[2] The related reducing agent sodium triethylborohydride is commercially available as toluene solutions.
LiBHEt3 is a stronger reducing agent than lithium borohydride and lithium aluminium hydride.
Preparation[]
LiBHEt3 is prepared by the reaction of lithium hydride (LiH) and triethylborane (Et3B) in tetrahydrofuran (THF):
- LiH + Et3B → LiEt3BH
Its THF solutions are stable indefinitely in the absence of moisture and air.
Reactions[]
Alkyl halides are reduced to the alkanes by LiBHEt3.[3][4][2]
LiBHEt3 reduces a wide range of functional groups, but so do many other hydride reagents. Instead, LiBHEt3 is reserved for difficult substrates, such as sterically hindered carbonyls, as illustrated by reduction of 2,2,4,4-tetramethyl-3-pentanone. Otherwise, it reduces acid anhydrides to alcohols and the carboxylic acid, not to the diol. Similarly lactones reduce to diols. α,β-Enones undergo 1,4-addition to give lithium enolates. Disulfides reduce to thiols (via thiolates). LiBHEt3 deprotonates carboxylic acids, but does not reduce the resulting lithium carboxylates. For similar reasons, epoxides undergo ring-opening upon treatment with LiBHEt3 to give the alcohol. With unsymmetrical epoxides, the reaction can proceed with high regio- and stereo- selectivity, favoring attack at the least hindered position:[2]
Acetals and ketals are not reduced by LiBHEt3. It can be used in the reductive cleavage of mesylates and tosylates.[5] LiBHEt3 can selectively deprotect tertiary N-acyl groups without affecting secondary amide functionality.[6] It has also shown to reduce aromatic esters to the corresponding alcohols as shown in eq 6 and 7.
LiBHEt3 also reduces pyridine and isoquinolines to piperidines and tetrahydroisoquinolines respectively.[7]
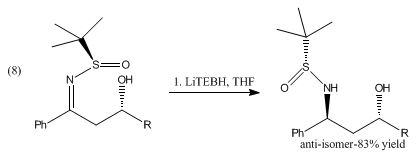
The reduction of β-hydroxysulfinyl imines with catecholborane and LiBHEt3 produces anti-1,3-amino alcohols shown in (8).[8]
Precautions[]
LiBHEt3 reacts exothermically, potentially violently, with water, alcohols, and acids, releasing hydrogen and the pyrophoric triethylborane.[2]
References[]
- ^ "Lithium triethylhydroborate". pubchem.ncbi.nlm.nih.gov. Retrieved 19 December 2021.
- ^ a b c Marek Zaidlewicz, Herbert C. Brown "Lithium Triethylborohydride" Encyclopedia of Reagents for Organic Synthesis 2001, John Wiley & Sons. doi:10.1002/047084289X.rl148
- ^ Marek Zaidlewicz, Herbert C. Brown (2001). "Lithium Triethylborohydride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rl148. ISBN 0471936235.CS1 maint: uses authors parameter (link)
- ^ Brown, H.C.; Kim, S.C.; Krishnamurthy, S. "Selective reductions. 27. Reaction of alkyl halides with representative complex metal hydrides and metal hydrides. Comparison of various hydride reducing agents" J. Org. Chem. 1980, 45, 1-12. doi:10.1021/jo01293a018
- ^ Baer, H.H.; Mekarska-Falicki, M. Can. J. Chem. 1985, 63, 3043.
- ^ Tanaka, H.; Ogasawara, K. Tetrahedron Lett. 2002, 43, 4417.
- ^ Blough, B.E.; Carroll, F. I. "Reduction of isoquinoline and pyridine-containing heterocycles with lithium triethylborohydride (Super-Hydride®)" Tetrahedron Lett. 1993, 34, 7239. doi:10.1016/S0040-4039(00)79297-5
- ^ Kochi, T; Tang, T.P.; Ellman, J.A. J. Am. Chem. Soc. 2002, 124, 6518.
- Borohydrides
- Lithium compounds
- Organoboranes
- Organolithium compounds
- Reducing agents