Lithium peroxide

| |
 __ Li+ __ O−
| |
| Names | |
|---|---|
| Other names
Dilithium peroxide, Lithium (I) peroxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.585 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Li2O2 | |
| Molar mass | 45.881 g/mol |
| Appearance | fine, white powder |
| Odor | odorless |
| Density | 2.31 g/cm3[1][2] |
| Melting point | Decomposes to Li2O at ~340°C [3] |
| Boiling point | NA |
| soluble | |
| Solubility | insoluble in alcohol |
| Structure | |
| hexagonal | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-13.82 kJ/g |
| Hazards | |
| GHS labelling: | |
 
| |
Signal word
|
Danger |
| H271, H272, H314 | |
| P210, P220, P221, P260, P264, P280, P283, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P306+P360, P310, P321, P363, P370+P378, P371+P380+P375, P405, P501 | |
| NFPA 704 (fire diamond) | 
3
0
2 OX |
| Related compounds | |
Other cations
|
Sodium peroxide Potassium peroxide Caesium peroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lithium peroxide is the inorganic compound with the formula Li2O2. It is a white, nonhygroscopic solid. Because of its high oxygen:mass and oxygen:volume ratios, the solid has been used to remove CO2 from the atmosphere in spacecraft.[4]
Preparation[]
It is prepared by the reaction of hydrogen peroxide and lithium hydroxide. This reaction initially produces lithium hydroperoxide:[4][5]
- LiOH + H2O2 → LiOOH + H2O
This lithium hydroperoxide has also been described as lithium peroxide monoperoxohydrate trihydrate (Li2O2·H2O2·3H2O). Dehydration of this material gives the anhydrous peroxide salt:
- 2 LiOOH → Li2O2 + H2O2
Li2O2 decomposes at about 450 °C to give lithium oxide:
- 2 Li2O2 → 2 Li2O + O2
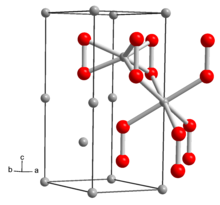
The structure of solid Li2O2 has been determined by X-ray crystallography and density functional theory. The solid features an eclipsed "ethane-like" Li6O2 subunits with an O-O distance of around 1.5 Å.[6]
Uses[]
It is used in air purifiers where weight is important, e.g., spacecraft to absorb carbon dioxide and release oxygen in the reaction:[4]
- 2 Li2O2 + 2 CO2 → 2 Li2CO3 + O2
It absorbs more CO2 than does the same weight of lithium hydroxide and offers the bonus of releasing oxygen.[7] Furthermore, unlike most other alkali metal peroxides, it is not hygroscopic.
The reversible lithium peroxide reaction is the basis for a prototype lithium–air battery. Using oxygen from the atmosphere allows the battery to eliminate storage of oxygen for its reaction, saving battery weight and size.[8]
The successful combination of a lithium-air battery overlain with an air-permeable mesh solar cell was announced by The Ohio State University in 2014.[9] The combination of two functions in one device (a "solar battery") is expected to reduce costs significantly compared to separate devices and controllers as are currently employed.
See also[]
References[]
- ^ "Physical Constants of Inorganic Compounds," in CRC Handbook of Chemistry and Physics, 91st Edition (Internet Version 2011), W. M. Haynes, ed., CRC Press/Taylor and Francis, Boca Raton, Florida. (pp: 4-72).
- ^ Speight, James G. (2005). Lange's Handbook of Chemistry (16th Edition). (pp: 1.40). McGraw-Hill. Online version available at: http://www.knovel.com/web/portal/browse/display?_EXT_KNOVEL_DISPLAY_bookid=1347&VerticalID=0
- ^ Phys.Chem.Chem.Phys.,2013,15, 11025. doi:10.1039/c3cp51056e
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 98. ISBN 978-0-08-022057-4.
- ^ E. Dönges "Lithium and Sodium Peroxides" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 979.
- ^ L. G. Cota and P. de la Mora "On the structure of lithium peroxide, Li2O2" Acta Crystallogr. 2005, vol. B61, pages 133-136. doi:10.1107/S0108768105003629
- ^ Ulrich Wietelmann, Richard J. Bauer "Lithium and Lithium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH: Weinheim. doi:10.1002/14356007.a15_393.pub2
- ^ Girishkumar, G.; B. McCloskey; AC Luntz; S. Swanson; W. Wilcke (July 2, 2010). "Lithium- air battery: Promise and challenges". The Journal of Physical Chemistry Letters. 1 (14): 2193–2203. doi:10.1021/jz1005384.
- ^ [1] Patent-pending device invented at The Ohio State University: the world’s first solar battery.
External links[]
- Peroxides
- Lithium compounds
- Oxidizing agents