Pedogenesis
Pedogenesis (from the Greek pedo-, or pedon, meaning 'soil, earth,' and genesis, meaning 'origin, birth') (also termed soil development, soil evolution, soil formation, and soil genesis) is the process of soil formation as regulated by the effects of place, environment, and history. Biogeochemical processes act to both create and destroy order (anisotropy) within soils. These alterations lead to the development of layers, termed soil horizons, distinguished by differences in color, structure, texture, and chemistry. These features occur in patterns of soil type distribution, forming in response to differences in soil forming factors.[1]
Pedogenesis is studied as a branch of pedology, the study of soil in its natural environment. Other branches of pedology are the study of soil morphology, and soil classification. The study of pedogenesis is important to understanding soil distribution patterns in current (soil geography) and past (paleopedology) geologic periods.
Overview[]
Soil develops through a series of changes.[2] The starting point is weathering of freshly accumulated parent material. Primitive microbes feed on simple compounds (nutrients) released by weathering, and produce acids which contribute to weathering. They also leave behind organic residues.[citation needed]
New soils increase in depth by a combination of weathering, and further deposition. An estimated 1/10 mm per year rate of soil production from weathering fits observations rates.[3] New soils can also deepen from dust deposition. Gradually soil is able to support higher forms of plants and animals, starting with pioneer species, and proceeding to more complex plant and animal communities. Soils deepen with accumulation of humus primarily due to the activities of higher plants. Topsoils deepen through soil mixing.[4] As soils mature, they develop layers as organic matter accumulates and leaching takes place. This development of layers is the beginning of the soil profile.[citation needed]
Factors of soil formation[]
Soil formation is influenced by at least five classic factors that are intertwined in the evolution of a soil. They are: parent material, climate, topography (relief), organisms, and time.[5] When reordered to climate, relief, organisms, parent material, and time, they form the acronym CROPT.[6]
Parent material[]
The mineral material from which a soil forms is called parent material. Rock, whether its origin is igneous, sedimentary, or metamorphic, is the source of all soil mineral materials and the origin of all plant nutrients with the exceptions of nitrogen, hydrogen and carbon. As the parent material is chemically and physically weathered, transported, deposited and precipitated, it is transformed into a soil.[citation needed]
Typical soil parent mineral materials are:[7]
- Quartz: SiO2
- Calcite: CaCO3
- Feldspar: KAlSi3O8
- Mica (biotite): K(Mg,Fe)
3(AlSi
3O
10)(F,OH)
2

Parent materials are classified according to how they came to be deposited. Residual materials are mineral materials that have weathered in place from primary bedrock. Transported materials are those that have been deposited by water, wind, ice or gravity. Cumulose material is organic matter that has grown and accumulates in place.[citation needed]
Residual soils are soils that develop from their underlying parent rocks and have the same general chemistry as those rocks. The soils found on mesas, plateaux, and plains are residual soils. In the United States as little as three percent of the soils are residual.[8]
Most soils derive from transported materials that have been moved many miles by wind, water, ice and gravity.
- Aeolian processes (movement by wind) are capable of moving silt and fine sand many hundreds of miles, forming loess soils (60–90 percent silt),[9] common in the Midwest of North America, north-western Europe, Argentina and Central Asia. Clay is seldom moved by wind as it forms stable aggregates.
- Water-transported materials are classed as either alluvial, lacustrine, or marine. Alluvial materials are those moved and deposited by flowing water. Sedimentary deposits settled in lakes are called lacustrine. Lake Bonneville and many soils around the Great Lakes of the United States are examples. Marine deposits, such as soils along the Atlantic and Gulf Coasts and in the Imperial Valley of California of the United States, are the beds of ancient seas that have been revealed as the land uplifted.[citation needed]
- Ice moves parent material and makes deposits in the form of terminal and lateral moraines in the case of stationary glaciers. Retreating glaciers leave smoother ground moraines and in all cases, outwash plains are left as alluvial deposits are moved downstream from the glacier.[citation needed]
- Parent material moved by gravity is obvious at the base of steep slopes as talus cones and is called colluvial material.[citation needed]
Cumulose parent material is not moved but originates from deposited organic material. This includes peat and muck soils and results from preservation of plant residues by the low oxygen content of a high water table. While peat may form sterile soils, muck soils may be very fertile.[citation needed]
Weathering[]
The weathering of parent material takes the form of physical weathering (disintegration), chemical weathering (decomposition) and chemical transformation. Generally, minerals that are formed under high temperatures and pressures at great depths within the Earth's mantle are less resistant to weathering, while minerals formed at low temperature and pressure environment of the surface are more resistant to weathering.[citation needed] Weathering is usually confined to the top few meters of geologic material, because physical, chemical, and biological stresses and fluctuations generally decrease with depth.[10] Physical disintegration begins as rocks that have solidified deep in the Earth are exposed to lower pressure near the surface and swell and become mechanically unstable. Chemical decomposition is a function of mineral solubility, the rate of which doubles with each 10 °C rise in temperature, but is strongly dependent on water to effect chemical changes. Rocks that will decompose in a few years in tropical climates will remain unaltered for millennia in deserts.[11] Structural changes are the result of hydration, oxidation, and reduction. Chemical weathering mainly results from the excretion of organic acids and chelating compounds by bacteria[12] and fungi,[13] thought to increase under present-day greenhouse effect.[14]
- Physical disintegration is the first stage in the transformation of parent material into soil. Temperature fluctuations cause expansion and contraction of the rock, splitting it along lines of weakness. Water may then enter the cracks and freeze and cause the physical splitting of material along a path toward the center of the rock, while temperature gradients within the rock can cause exfoliation of "shells". Cycles of wetting and drying cause soil particles to be abraded to a finer size, as does the physical rubbing of material as it is moved by wind, water, and gravity. Water can deposit within rocks minerals that expand upon drying, thereby stressing the rock. Finally, organisms reduce parent material in size and create crevices and pores through the mechanical action of plant roots and the digging activity of animals.[15] Grinding of parent material by rock-eating animals also contributes to incipient soil formation.[16]
- Chemical decomposition and structural changes result when minerals are made soluble by water or are changed in structure. The first three of the following list are solubility changes and the last three are structural changes.[17]
- The solution of salts in water results from the action of bipolar water molecules on ionic salt compounds producing a solution of ions and water, removing those minerals and reducing the rock's integrity, at a rate depending on water flow and pore channels.[18]
- Hydrolysis is the transformation of minerals into polar molecules by the splitting of intervening water. This results in soluble acid-base pairs. For example, the hydrolysis of orthoclase-feldspar transforms it to acid silicate clay and basic potassium hydroxide, both of which are more soluble.[19]
- In carbonation, the solution of carbon dioxide in water forms carbonic acid. Carbonic acid will transform calcite into more soluble calcium bicarbonate.[20]
- Hydration is the inclusion of water in a mineral structure, causing it to swell and leaving it stressed and easily decomposed.[21]
- Oxidation of a mineral compound is the inclusion of oxygen in a mineral, causing it to increase its oxidation number and swell due to the relatively large size of oxygen, leaving it stressed and more easily attacked by water (hydrolysis) or carbonic acid (carbonation).[22]
- Reduction, the opposite of oxidation, means the removal of oxygen, hence the oxidation number of some part of the mineral is reduced, which occurs when oxygen is scarce. The reduction of minerals leaves them electrically unstable, more soluble and internally stressed and easily decomposed. It mainly occurs in waterlogged conditions.[23]
Of the above, hydrolysis and carbonation are the most effective, in particular in regions of high rainfall, temperature and physical erosion.[24] Chemical weathering becomes more effective as the surface area of the rock increases, thus is favoured by physical disintegration.[25] This stems in latitudinal and altitudinal climate gradients in regolith formation.[26][27]
Saprolite is a particular example of a residual soil formed from the transformation of granite, metamorphic and other types of bedrock into clay minerals. Often called [weathered granite], saprolite is the result of weathering processes that include: hydrolysis, chelation from organic compounds, hydration (the solution of minerals in water with resulting cation and anion pairs) and physical processes that include freezing and thawing. The mineralogical and chemical composition of the primary bedrock material, its physical features, including grain size and degree of consolidation, and the rate and type of weathering transforms the parent material into a different mineral. The texture, pH and mineral constituents of saprolite are inherited from its parent material. This process is also called arenization, resulting in the formation of sandy soils (granitic arenas), thanks to the much higher resistance of quartz compared to other mineral components of granite (micas, amphiboles, feldspars).[28]
Climate[]
The principal climatic variables influencing soil formation are effective precipitation (i.e., precipitation minus evapotranspiration) and temperature, both of which affect the rates of chemical, physical, and biological processes. Temperature and moisture both influence the organic matter content of soil through their effects on the balance between primary production and decomposition: the colder or drier the climate the lesser atmospheric carbon is fixed as organic matter while the lesser organic matter is decomposed.[29]
Climate is the dominant factor in soil formation, and soils show the distinctive characteristics of the climate zones in which they form, with a feedback to climate through transfer of carbon stocked in soil horizons back to the atmosphere.[30] If warm temperatures and abundant water are present in the profile at the same time, the processes of weathering, leaching, and plant growth will be maximized. According to the climatic determination of biomes, humid climates favor the growth of trees. In contrast, grasses are the dominant native vegetation in subhumid and semiarid regions, while shrubs and brush of various kinds dominate in arid areas.[31]
Water is essential for all the major chemical weathering reactions. To be effective in soil formation, water must penetrate the regolith. The seasonal rainfall distribution, evaporative losses, site topography, and soil permeability interact to determine how effectively precipitation can influence soil formation. The greater the depth of water penetration, the greater the depth of weathering of the soil and its development. Surplus water percolating through the soil profile transports soluble and suspended materials from the upper layers (eluviation) to the lower layers (illuviation), including clay particles[32] and dissolved organic matter.[33] It may also carry away soluble materials in the surface drainage waters. Thus, percolating water stimulates weathering reactions and helps differentiate soil horizons. Likewise, a deficiency of water is a major factor in determining the characteristics of soils of dry regions. Soluble salts are not leached from these soils, and in some cases they build up to levels that curtail plant[34] and microbial growth.[35] Soil profiles in arid and semi-arid regions are also apt to accumulate carbonates and certain types of expansive clays (calcrete or caliche horizons).[36][37] In tropical soils, when the soil has been deprived of vegetation (e.g. by deforestation) and thereby is submitted to intense evaporation, the upward capillary movement of water, which has dissolved iron and aluminum salts, is responsible for the formation of a superficial hard pan of laterite or bauxite, respectively, which is improper for cultivation, a known case of irreversible soil degradation (lateritization, bauxitization).[38]
The direct influences of climate include:[39]
- A shallow accumulation of lime in low rainfall areas as caliche
- Formation of acid soils in humid areas
- Erosion of soils on steep hillsides
- Deposition of eroded materials downstream
- Very intense chemical weathering, leaching, and erosion in warm and humid regions where soil does not freeze
Climate directly affects the rate of weathering and leaching. Wind moves sand and smaller particles (dust), especially in arid regions where there is little plant cover, depositing it close[40] or far from the entrainment source.[41] The type and amount of precipitation influence soil formation by affecting the movement of ions and particles through the soil, and aid in the development of different soil profiles. Soil profiles are more distinct in wet and cool climates, where organic materials may accumulate, than in wet and warm climates, where organic materials are rapidly consumed.[42] The effectiveness of water in weathering parent rock material depends on seasonal and daily temperature fluctuations, which favour tensile stresses in rock minerals, and thus their mechanical disaggregation, a process called thermal fatigue.[43] By the same process freeze-thaw cycles are an effective mechanism which breaks up rocks and other consolidated materials.[44]
Climate also indirectly influences soil formation through the effects of vegetation cover and biological activity, which modify the rates of chemical reactions in the soil.[45]
Topography[]
The topography, or relief, is characterized by the inclination (slope), elevation, and orientation of the terrain. Topography determines the rate of precipitation or runoff and rate of formation or erosion of the surface soil profile. The topographical setting may either hasten or retard the work of climatic forces.[citation needed]
Steep slopes encourage rapid soil loss by erosion and allow less rainfall to enter the soil before running off and hence, little mineral deposition in lower profiles. In semiarid regions, the lower effective rainfall on steeper slopes also results in less complete vegetative cover, so there is less plant contribution to soil formation. For all of these reasons, steep slopes prevent the formation of soil from getting very far ahead of soil destruction. Therefore, soils on steep terrain tend to have rather shallow, poorly developed profiles in comparison to soils on nearby, more level sites.[46]
Soils at the bottom of a hill will get more water than soils on the slopes, and soils on the slopes that face the sun's path will be drier than soils on slopes that do not. Topography determines exposure to weather, fire, and other forces of man and nature. Mineral accumulations, plant nutrients, type of vegetation, vegetation growth, erosion, and water drainage are dependent on topographic relief.[citation needed]
In swales and depressions where runoff water tends to concentrate, the regolith is usually more deeply weathered and soil profile development is more advanced. However, in the lowest landscape positions, water may saturate the regolith to such a degree that drainage and aeration are restricted. Here, the weathering of some minerals and the decomposition of organic matter are retarded, while the loss of iron and manganese is accelerated. In such low-lying topography, special profile features characteristic of wetland soils may develop. Depressions allow the accumulation of water, minerals and organic matter and in the extreme, the resulting soils will be saline marshes or peat bogs. Intermediate topography affords the best conditions for the formation of an agriculturally productive soil.[citation needed]
Recurring patterns of topography result in toposequences or soil catenas. These patterns emerge from topographic differences in erosion, deposition, fertility, soil moisture, plant cover, other soil biology, fire-history, and exposure to the elements. These same differences are important to understanding natural history and when managing the land resource.[citation needed]
Organisms[]
Each soil has a unique combination of microbial, plant, animal and human influences acting upon it. Microorganisms are particularly influential in the mineral transformations critical to the soil forming process. Additionally, some bacteria can fix atmospheric nitrogen and some fungi are efficient at extracting deep soil phosphorus and increasing soil carbon levels in the form of glomalin. Plants hold soil against erosion, and accumulated plant material build soil humus levels. Plant root exudation supports microbial activity. Animals serve to decompose plant materials and mix soil through bioturbation.[citation needed]
Soil is the most abundant ecosystem on Earth, but the vast majority of organisms in soil are microbes, a great many of which have not been described.[47][48] There may be a population limit of around one billion cells per gram of soil, but estimates of the number of species vary widely from 50,000 per gram to over a million per gram of soil.[47][49] The total number of organisms and species can vary widely according to soil type, location, and depth.[48][49]
Plants, animals, fungi, bacteria and humans affect soil formation (see soil biomantle and stonelayer). Soil animals, including soil macrofauna and soil mesofauna, mix soils as they form burrows and pores, allowing moisture and gases to move about, a process called bioturbation.[50] In the same way, plant roots penetrate soil horizons and open channels upon decomposition.[51] Plants with deep taproots can penetrate many metres through the different soil layers to bring up nutrients from deeper in the profile.[52] Plants have fine roots that excrete organic compounds (sugars, organic acids, mucigel), slough off cells (in particular at their tip) and are easily decomposed, adding organic matter to soil, a process called rhizodeposition.[53] Micro-organisms, including fungi and bacteria, effect chemical exchanges between roots and soil and act as a reserve of nutrients in a soil biological hotspot called rhizosphere.[54] The growth of roots through the soil stimulates microbial populations, stimulating in turn the activity of their predators (notably amoeba), thereby increasing the mineralization rate, and in last turn root growth, a positive feedback called the soil microbial loop.[55] Out of root influence, in the bulk soil, most bacteria are in a quiescent stage, forming microaggregates, i.e. mucilaginous colonies to which clay particles are glued, offering them a protection against desiccation and predation by soil microfauna (bacteriophagous protozoa and nematodes).[56] Microaggregates (20-250 μm) are ingested by soil mesofauna and macrofauna, and bacterial bodies are partly or totally digested in their guts.[57]
Humans impact soil formation by removing vegetation cover with erosion, waterlogging, lateritization or podzolization (according to climate and topography) as the result.[58] Their tillage also mixes the different soil layers, restarting the soil formation process as less weathered material is mixed with the more developed upper layers, resulting in net increased rate of mineral weathering.[59]
Earthworms, ants, termites, moles, gophers, as well as some millipedes and tenebrionid beetles mix the soil as they burrow, significantly affecting soil formation.[60] Earthworms ingest soil particles and organic residues, enhancing the availability of plant nutrients in the material that passes through their bodies.[61] They aerate and stir the soil and create stable soil aggregates, after having disrupted links between soil particles during the intestinal transit of ingested soil,[62] thereby assuring ready infiltration of water.[63] In addition, as ants and termites build mounds, they transport soil materials from one horizon to another.[64] Other important functions are fulfilled by earthworms in the soil ecosystem, in particular their intense mucus production, both within the intestine and as a lining in their galleries,[65] exert a priming effect on soil microflora,[66] giving them the status of ecosystem engineers, which they share with ants and termites.[67]
In general, the mixing of the soil by the activities of animals, sometimes called pedoturbation, tends to undo or counteract the tendency of other soil-forming processes that create distinct horizons.[68] Termites and ants may also retard soil profile development by denuding large areas of soil around their nests, leading to increased loss of soil by erosion.[69] Large animals such as gophers, moles, and prairie dogs bore into the lower soil horizons, bringing materials to the surface.[70] Their tunnels are often open to the surface, encouraging the movement of water and air into the subsurface layers. In localized areas, they enhance mixing of the lower and upper horizons by creating, and later refilling the tunnels. Old animal burrows in the lower horizons often become filled with soil material from the overlying A horizon, creating profile features known as crotovinas.[71]
Vegetation impacts soils in numerous ways. It can prevent erosion caused by excessive rain that might result from surface runoff.[72] Plants shade soils, keeping them cooler[73] and slow evaporation of soil moisture,[74] or conversely, by way of transpiration, plants can cause soils to lose moisture, resulting in complex and highly variable relationships between leaf area index (measuring light interception) and moisture loss: more generally plants prevent soil from desiccation during driest months while they dry it during moister months, thereby acting as a buffer against strong moisture variation.[75] Plants can form new chemicals that can break down minerals, both directly[76] and indirectly through mycorrhizal fungi[13] and rhizosphere bacteria,[77] and improve the soil structure.[78] The type and amount of vegetation depends on climate, topography, soil characteristics and biological factors, mediated or not by human activities.[79][80] Soil factors such as density, depth, chemistry, pH, temperature and moisture greatly affect the type of plants that can grow in a given location. Dead plants and fallen leaves and stems begin their decomposition on the surface. There, organisms feed on them and mix the organic material with the upper soil layers; these added organic compounds become part of the soil formation process.[81]
The influence of man, and by association, fire, are state factors placed within the organisms state factor.[82] Man can import, or extract, nutrients and energy in ways that dramatically change soil formation. Accelerated soil erosion due to overgrazing, and Pre-Columbian terraforming the Amazon basin resulting in Terra Preta are two examples of the effects of man's management.
Human activities widely influence soil formation.[83] For example, it is believed that Native Americans regularly set fires to maintain several large areas of prairie grasslands in Indiana and Michigan, although climate and mammalian grazers (e.g. bisons) are also advocated to explain the maintenance of the Great Plains of North America.[84] In more recent times, human destruction of natural vegetation and subsequent tillage of the soil for crop production has abruptly modified soil formation.[85] Likewise, irrigating soil in an arid region drastically influences soil-forming factors,[86] as does adding fertilizer and lime to soils of low fertility.[87]
Distinct ecosystems produce distinct soils, sometimes in easily observable ways. For example, three species of land snails in the genus Euchondrus in the Negev desert are noted for eating lichens growing under the surface limestone rocks and slabs (endolithic lichens).[88] They disrupt and eat the limestone.[88] Their grazing results in the weathering of the stones, and the subsequent formation of soil.[88] They have a significant effect on the region: the total population of snails is estimated to process between 0.7 and 1.1 metric ton per hectare per year of limestone in the Negev desert.[88]
The effects of ancient ecosystems are not as easily observed, and this challenges the understanding of soil formation. For example, the chernozems of the North American tallgrass prairie have a humus fraction nearly half of which is charcoal. This outcome was not anticipated because the antecedent prairie fire ecology capable of producing these distinct deep rich black soils is not easily observed.[89]
Time[]
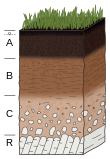
Time is a factor in the interactions of all the above.[5] While a mixture of sand, silt and clay constitute the texture of a soil and the aggregation of those components produces peds, the development of a distinct B horizon marks the development of a soil or pedogenesis.[90] With time, soils will evolve features that depend on the interplay of the prior listed soil-forming factors.[5] It takes decades[91] to several thousand years for a soil to develop a profile,[92] although the notion of soil development has been criticized, soil being in a constant state-of-change under the influence of fluctuating soil-forming factors.[93] That time period depends strongly on climate, parent material, relief, and biotic activity.[94][95] For example, recently deposited material from a flood exhibits no soil development as there has not been enough time for the material to form a structure that further defines soil.[96] The original soil surface is buried, and the formation process must begin anew for this deposit. Over time the soil will develop a profile that depends on the intensities of biota and climate. While a soil can achieve relative stability of its properties for extended periods,[92] the soil life cycle ultimately ends in soil conditions that leave it vulnerable to erosion.[97] Despite the inevitability of soil retrogression and degradation, most soil cycles are long.[92]
Soil-forming factors continue to affect soils during their existence, even on "stable" landscapes that are long-enduring, some for millions of years.[92] Materials are deposited on top[98] or are blown or washed from the surface.[99] With additions, removals and alterations, soils are always subject to new conditions. Whether these are slow or rapid changes depends on climate, topography and biological activity.[100]
Time as a soil-forming factor may be investigated by studying soil chronosequences, in which soils of different ages but with minor differences in other soil-forming factors can be compared.[101]
Paleosols are soils formed during previous soil forming conditions.
History of research[]

Dokuchaev's equation[]
Russian geologist Vasily Dokuchaev, commonly regarded as the father of pedology, determined in 1883[102] that soil formation occurs over time under the influence of climate, vegetation, topography, and parent material. He demonstrated this in 1898 using the soil forming equation:[103]
- soil = f(cl, o, p) tr
(where cl or c = climate, o = organisms, p = biological processes) tr = relative time (young, mature, old)
Hans Jenny's state equation[]
American soil scientist Hans Jenny published in 1941 a state equation for the factors influencing soil formation:
- S = f(cl, o, r, p, t, …)
- S soil formation
- cl (sometimes c) climate
- o organisms (soil microbiology, soil mesofauna, soil biology)
- r relief
- p parent material
- t time
This is often remembered with the mnemonic Clorpt.
Jenny's state equation in Factors of Soil Formation differs from the Vasily Dokuchaev equation, treating time (t) as a factor, adding topographic relief (r), and pointedly leaving the ellipsis "open" for more factors (state variables) to be added as our understanding becomes more refined.
There are two principal methods that the state equation may be solved: first in a theoretical or conceptual manner by logical deductions from certain premises, and second empirically by experimentation or field observation. The empirical method is still mostly employed today, and soil formation can be defined by varying a single factor and keeping the other factors constant. This led to the development of empirical models to describe pedogenesis, such as climofunctions, biofunctions, topofunctions, lithofunctions, and chronofunctions. Since Hans Jenny published his formulation in 1941, it has been used by innumerable soil surveyors all over the world as a qualitative list for understanding the factors that may be important for producing the soil pattern within a region.[104]
Soil forming processes[]
Soils develop from parent material by various weathering processes. Organic matter accumulation, decomposition, and humification are as critically important to soil formation as weathering. The zone of humification and weathering is termed the solum.[citation needed]
Soil acidification resulting from soil respiration supports chemical weathering. Plants contribute to chemical weathering through root exudates.[citation needed]
Soils can be enriched by deposition of sediments on floodplains and alluvial fans, and by wind-borne deposits.[citation needed]
Soil mixing (pedoturbation) is often an important factor in soil formation. Pedoturbation includes churning clays, cryoturbation, and bioturbation. Types of bioturbation include faunal pedoturbation (animal burrowing), floral pedoturbation (root growth, tree-uprootings), and fungal pedoturbation (mycelia growth). Pedoturbation transforms soils through destratification, mixing, and sorting, as well as creating preferential flow paths for soil gas and infiltrating water. The zone of active bioturbation is termed the soil biomantle.[citation needed]
Soil moisture content and water flow through the soil profile support leaching of soluble constituents, and eluviation. Eluviation is the translocation of colloid material, such as organic matter, clay and other mineral compounds. Transported constituents are deposited due to differences in soil moisture and soil chemistry, especially soil pH and redox potential. The interplay of removal and deposition results in contrasting soil horizons.[citation needed]
Key soil forming processes especially important to macro-scale patterns of soil formation are:[105]
- Laterization
- Podsolization
- Calcification
- Salinization
- Gleization
Examples[]
A variety of mechanisms contribute to soil formation, including siltation, erosion, overpressure and lake bed succession. A specific example of the evolution of soils in prehistoric lake beds is in the Makgadikgadi Pans of the Kalahari Desert, where change in an ancient river course led to millennia of salinity buildup and formation of calcretes and silcretes.[106]
Notes[]
- ^ Buol, S. W.; Hole, F. D. & McCracken, R. J. (1973). Soil Genesis and Classification (First ed.). Ames, IA: Iowa State University Press. ISBN 978-0-8138-1460-5.
- ^ Jenny, Hans (1994). Factors of soil formation: A System of Quantitative Pedology (PDF). New York: Dover. ISBN 978-0-486-68128-3. Archived from the original (PDF) on 25 February 2013. Retrieved 4 September 2014.
- ^ Scalenghe, R., Territo, C., Petit, S., Terribile, F., Righi, D. (2016). "The role of pedogenic overprinting in the obliteration of parent material in some polygenetic landscapes of Sicily (Italy)". Geoderma Regional. 7: 49–58. doi:10.1016/j.geodrs.2016.01.003.CS1 maint: multiple names: authors list (link)
- ^ Wilkinson, M.T., Humpreys, G.S. (2005). "Exploring pedogenesis via nuclide-based soil production rates and OSL-based bioturbation rates". Australian Journal of Soil Research. 43 (6): 767. doi:10.1071/SR04158.CS1 maint: multiple names: authors list (link)
- ^ Jump up to: a b c Jenny, Hans (1941). Factors of soil formation: a system of qunatitative pedology (PDF). New York: McGraw-Hill. Archived from the original (PDF) on 8 August 2017. Retrieved 17 December 2017.
- ^ Ritter, Michael E. "The physical environment: an introduction to physical geography". Retrieved 17 December 2017.
- ^ Donahue, Miller & Shickluna 1977, pp. 20–21.
- ^ Donahue, Miller & Shickluna 1977, p. 21.
- ^ Donahue, Miller & Shickluna 1977, p. 24.
- ^ "Weathering". University of Regina. Retrieved 17 December 2017.
- ^ Gilluly, James; Waters, Aaron Clement & Woodford, Alfred Oswald (1975). Principles of geology (4th ed.). San Francisco: W.H. Freeman. ISBN 978-0-7167-0269-6.
- ^ Uroz, Stéphane; Calvaruso, Christophe; Turpault, Marie-Pierre & Frey-Klett, Pascale (2009). "Mineral weathering by bacteria: ecology, actors and mechanisms". Trends in Microbiology. 17 (8): 378–87. doi:10.1016/j.tim.2009.05.004. PMID 19660952.
- ^ Jump up to: a b Landeweert, Renske; Hoffland, Ellis; Finlay, Roger D.; Kuyper, Thom W. & Van Breemen, Nico (2001). "Linking plants to rocks: ectomycorrhizal fungi mobilize nutrients from minerals". Trends in Ecology and Evolution. 16 (5): 248–54. doi:10.1016/S0169-5347(01)02122-X. PMID 11301154.
- ^ Andrews, Jeffrey A. & Schlesinger, William H. (2001). "Soil CO2 dynamics, acidification, and chemical weathering in a temperate forest with experimental CO2 enrichment". Global Biogeochemical Cycles. 15 (1): 149–62. Bibcode:2001GBioC..15..149A. doi:10.1029/2000GB001278.
- ^ Donahue, Miller & Shickluna 1977, pp. 28–31.
- ^ Jones, Clive G. & Shachak, Moshe (1990). "Fertilization of the desert soil by rock-eating snails" (PDF). Nature. 346 (6287): 839–41. Bibcode:1990Natur.346..839J. doi:10.1038/346839a0. S2CID 4311333. Retrieved 17 December 2017.
- ^ Donahue, Miller & Shickluna 1977, pp. 31–33.
- ^ Li, Li; Steefel, Carl I. & Yang, Li (2008). "Scale dependence of mineral dissolution rates within single pores and fractures" (PDF). Geochimica et Cosmochimica Acta. 72 (2): 360–77. Bibcode:2008GeCoA..72..360L. doi:10.1016/j.gca.2007.10.027. Archived from the original (PDF) on 1 November 2015. Retrieved 17 December 2017.
- ^ La Iglesia, Ángel; Martin-Vivaldi Jr, Juan Luis & López Aguayo, Francisco (1976). "Kaolinite crystallization at room temperature by homogeneous precipitation. III. Hydrolysis of feldspars" (PDF). Clays and Clay Minerals. 24 (6287): 36–42. Bibcode:1990Natur.346..839J. doi:10.1038/346839a0. S2CID 4311333. Archived from the original (PDF) on 9 August 2017. Retrieved 17 December 2017.
- ^ Al-Hosney, Hashim & Grassian, Vicki H. (2004). "Carbonic acid: an important intermediate in the surface chemistry of calcium carbonate". Journal of the American Chemical Society. 126 (26): 8068–69. doi:10.1021/ja0490774. PMID 15225019.
- ^ Jiménez-González, Inmaculada; Rodríguez‐Navarro, Carlos & Scherer, George W. (2008). "Role of clay minerals in the physicomechanical deterioration of sandstone". Journal of Geophysical Research. 113 (F02021): 1–17. Bibcode:2008JGRF..113.2021J. doi:10.1029/2007JF000845.
- ^ Mylvaganam, Kausala & Zhang, Liangchi (2002). "Effect of oxygen penetration in silicon due to nano-indentation" (PDF). Nanotechnology. 13 (5): 623–26. Bibcode:2002Nanot..13..623M. doi:10.1088/0957-4484/13/5/316. Retrieved 17 December 2017.
- ^ Favre, Fabienne; Tessier, Daniel; Abdelmoula, Mustapha; Génin, Jean-Marie; Gates, Will P. & Boivin, Pascal (2002). "Iron reduction and changes in cation exchange capacity in intermittently waterlogged soil". European Journal of Soil Science. 53 (2): 175–83. doi:10.1046/j.1365-2389.2002.00423.x.
- ^ Riebe, Clifford S.; Kirchner, James W. & Finkel, Robert C. (2004). "Erosional and climatic effects on long-term chemical weathering rates in granitic landscapes spanning diverse climate regimes" (PDF). Earth and Planetary Science Letters. 224 (3/4): 547–62. Bibcode:2004E&PSL.224..547R. doi:10.1016/j.epsl.2004.05.019. Retrieved 17 December 2017.
- ^ "Rates of weathering" (PDF). Retrieved 17 December 2017.
- ^ Dere, Ashlee L.; White, Timothy S.; April, Richard H.; Reynolds, Bryan; Miller, Thomas E.; Knapp, Elizabeth P.; McKay, Larry D. & Brantley, Susan L. (2013). "Climate dependence of feldspar weathering in shale soils along a latitudinal gradient". Geochimica et Cosmochimica Acta. 122: 101–26. Bibcode:2013GeCoA.122..101D. doi:10.1016/j.gca.2013.08.001.
- ^ Kitayama, Kanehiro; Majalap-Lee, Noreen & Aiba, Shin-ichiro (2000). "Soil phosphorus fractionation and phosphorus-use efficiencies of tropical rainforests along altitudinal gradients of Mount Kinabalu, Borneo". Oecologia. 123 (3): 342–49. Bibcode:2000Oecol.123..342K. doi:10.1007/s004420051020. PMID 28308588. S2CID 20660989.
- ^ Sequeira Braga, Maria Amália; Paquet, Hélène & Begonha, Arlindo (2002). "Weathering of granites in a temperate climate (NW Portugal): granitic saprolites and arenization" (PDF). Catena. 49 (1/2): 41–56. doi:10.1016/S0341-8162(02)00017-6. Retrieved 17 December 2017.
- ^ Epstein, Howard E.; Burke, Ingrid C. & Lauenroth, William K. (2002). "Regional patterns of decomposition and primary production rates in the U.S. Great Plains" (PDF). Ecology. 83 (2): 320–27. doi:10.1890/0012-9658(2002)083[0320:RPODAP]2.0.CO;2. Retrieved 17 December 2017.
- ^ Davidson, Eric A. & Janssens, Ivan A. (2006). "Temperature sensitivity of soil carbon decomposition and feedbacks to climate change" (PDF). Nature. 440 (9 March 2006): 165‒73. Bibcode:2006Natur.440..165D. doi:10.1038/nature04514. PMID 16525463. S2CID 4404915. Retrieved 17 December 2017.
- ^ Woodward, F. Ian; Lomas, Mark R. & Kelly, Colleen K. (2004). "Global climate and the distribution of plant biomes". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 359 (1450): 1465–76. doi:10.1098/rstb.2004.1525. PMC 1693431. PMID 15519965.
- ^ Fedoroff, Nicolas (1997). "Clay illuviation in Red Mediterranean soils". Catena. 28 (3/4): 171–89. doi:10.1016/S0341-8162(96)00036-7.
- ^ Michalzik, Beate; Kalbitz, Karsten; Park, Ji-Hyung; Solinger, Stephan & Matzner, Egbert (2001). "Fluxes and concentrations of dissolved organic carbon and nitrogen: a synthesis for temperate forests" (PDF). Biogeochemistry. 52 (2): 173–205. doi:10.1023/A:1006441620810. S2CID 97298438. Retrieved 17 December 2017.
- ^ Bernstein, Leon (1975). "Effects of salinity and sodicity on plant growth". Annual Review of Phytopathology. 13: 295–312. doi:10.1146/annurev.py.13.090175.001455.
- ^ Yuan, Bing-Cheng; Li, Zi-Zhen; Liu, Hua; Gao, Meng & Zhang, Yan-Yu (2007). "Microbial biomass and activity in salt affected soils under arid conditions" (PDF). Applied Soil Ecology. 35 (2): 319–28. doi:10.1016/j.apsoil.2006.07.004. Retrieved 17 December 2017.
- ^ Schlesinger, William H. (1982). "Carbon storage in the caliche of arid soils: a case study from Arizona" (PDF). Soil Science. 133 (4): 247–55. doi:10.1146/annurev.py.13.090175.001455. Archived from the original (PDF) on 4 March 2018. Retrieved 17 December 2017.
- ^ Nalbantoglu, Zalihe & Gucbilmez, Emin (2001). "Improvement of calcareous expansive soils in semi-arid environments". Journal of Arid Environments. 47 (4): 453–63. Bibcode:2001JArEn..47..453N. doi:10.1006/jare.2000.0726.
- ^ Retallack, Gregory J. (2010). "Lateritization and bauxitization events" (PDF). Economic Geology. 105 (3): 655–67. doi:10.2113/gsecongeo.105.3.655. Retrieved 17 December 2017.
- ^ Donahue, Miller & Shickluna 1977, p. 35.
- ^ Pye, Kenneth & Tsoar, Haim (1987). "The mechanics and geological implications of dust transport and deposition in deserts with particular reference to loess formation and dune sand diagenesis in the northern Negev, Israel" (PDF). In Frostick, Lynne & Reid, Ian (eds.). Desert sediments: ancient and modern. Geological Society of London, Special Publications. 35. pp. 139–56. Bibcode:1987GSLSP..35..139P. doi:10.1144/GSL.SP.1987.035.01.10. ISBN 978-0-632-01905-2. S2CID 128746705. Retrieved 17 December 2017.
- ^ Prospero, Joseph M. (1999). "Long-range transport of mineral dust in the global atmosphere: impact of African dust on the environment of the southeastern United States". Proceedings of the National Academy of Sciences of the United States of America. 96 (7): 3396–403. Bibcode:1999PNAS...96.3396P. doi:10.1073/pnas.96.7.3396. PMC 34280. PMID 10097049.
- ^ Post, Wilfred M.; Emanuel, William R.; Zinke, Paul J. & Stangerberger, Alan G. (1999). "Soil carbon pools and world life zones". Nature. 298 (5870): 156–59. Bibcode:1982Natur.298..156P. doi:10.1038/298156a0. S2CID 4311653.
- ^ Gómez-Heras, Miguel; Smith, Bernard J. & Fort, Rafael (2006). "Surface temperature differences between minerals in crystalline rocks: implications for granular disaggregation of granites through thermal fatigue". Geomorphology. 78 (3/4): 236–49. Bibcode:2006Geomo..78..236G. doi:10.1016/j.geomorph.2005.12.013.
- ^ Nicholson, Dawn T. & Nicholson, Frank H. (2000). "Physical deterioration of sedimentary rocks subjected to experimental freeze–thaw weathering" (PDF). Earth Surface Processes and Landforms. 25 (12): 1295–307. Bibcode:2000ESPL...25.1295N. doi:10.1002/1096-9837(200011)25:12<1295::AID-ESP138>3.0.CO;2-E.
- ^ Lucas, Yves (2001). "The role of plants in controlling rates and products of weathering: importance of biological pumping" (PDF). Annual Review of Earth and Planetary Sciences. 29: 135–63. Bibcode:2001AREPS..29..135L. doi:10.1146/annurev.earth.29.1.135. Retrieved 17 December 2017.
- ^ Liu, Baoyuan; Nearing, Mark A. & Risse, L. Mark (1994). "Slope gradient effects on soil loss for steep slopes" (PDF). Transactions of the American Society of Agricultural and Biological Engineers. 37 (6): 1835–40. doi:10.13031/2013.28273. Retrieved 17 December 2017.
- ^ Jump up to: a b Gans, Jason; Wolinsky, Murray & Dunbar, John (2005). "Computational improvements reveal great bacterial diversity and high metal toxicity in soil" (PDF). Science. 309 (5739): 1387–90. Bibcode:2005Sci...309.1387G. doi:10.1126/science.1112665. PMID 16123304. S2CID 130269020. Retrieved 17 December 2017.
- ^ Jump up to: a b Dance, Amber (2008). "What lies beneath" (PDF). Nature. 455 (7214): 724–25. doi:10.1038/455724a. PMID 18843336. S2CID 30863755. Retrieved 17 December 2017.
- ^ Jump up to: a b Roesch, Luiz F.W.; Fulthorpe, Roberta R.; Riva, Alberto; Casella, George; Hadwin, Alison K.M.; Kent, Angela D.; Daroub, Samira H.; Camargo, Flavio A.O.; Farmerie, William G. & Triplett, Eric W. (2007). "Pyrosequencing enumerates and contrasts soil microbial diversity". The ISME Journal. 1 (4): 283–90. doi:10.1038/ismej.2007.53. PMC 2970868. PMID 18043639.
- ^ Meysman, Filip J.R.; Middelburg, Jack J. & Heip, Carlo H.R. (2006). "Bioturbation: a fresh look at Darwin's last idea" (PDF). Trends in Ecology and Evolution. 21 (12): 688–95. doi:10.1016/j.tree.2006.08.002. PMID 16901581. Retrieved 17 December 2017.
- ^ Williams, Stacey M. & Weil, Ray R. (2004). "Crop cover root channels may alleviate soil compaction effects on soybean crop" (PDF). Soil Science Society of America Journal. 68 (4): 1403–09. Bibcode:2004SSASJ..68.1403W. doi:10.2136/sssaj2004.1403. Retrieved 17 December 2017.
- ^ Lynch, Jonathan (1995). "Root architecture and plant productivity". Plant Physiology. 109 (1): 7–13. doi:10.1104/pp.109.1.7. PMC 157559. PMID 12228579.
- ^ Nguyen, Christophe (2003). "Rhizodeposition of organic C by plants: mechanisms and controls" (PDF). Agronomie. 23 (5/6): 375–96. doi:10.1051/agro:2003011. Retrieved 17 December 2017.
- ^ Widmer, Franco; Pesaro, Manuel; Zeyer, Josef & Blaser, Peter (2000). "Preferential flow paths: biological 'hot spots' in soils" (PDF). In Bundt, Maya (ed.). Highways through the soil: properties of preferential flow paths and transport of reactive compounds (Thesis). Zurich: ETH Library. pp. 53–75. doi:10.3929/ethz-a-004036424. hdl:20.500.11850/144808. Retrieved 17 December 2017.
- ^ Bonkowski, Michael (2004). "Protozoa and plant growth: the microbial loop in soil revisited". New Phytologist. 162 (3): 617–31. doi:10.1111/j.1469-8137.2004.01066.x. PMID 33873756.
- ^ Six, Johan; Bossuyt, Heleen; De Gryze, Steven & Denef, Karolien (2004). "A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics". Soil and Tillage Research. 79 (1): 7–31. doi:10.1016/j.still.2004.03.008.
- ^ Saur, Étienne & Ponge, Jean-François (1988). "Alimentary studies on the collembolan Paratullbergia callipygos using transmission electron microscopy" (PDF). Pedobiologia. 31 (5/6): 355–79. Retrieved 17 December 2017.
- ^ Oldeman, L. Roel (1992). "Global extent of soil degradation" (PDF). ISRIC Bi-Annual Report 1991/1992. Wagenngen, The Netherlands: ISRIC. pp. 19–36. Retrieved 17 December 2017.
- ^ Karathanasis, Anastasios D. & Wells, Kenneth L. (2004). "A comparison of mineral weathering trends between two management systems on a catena of loess-derived soils". Soil Science Society of America Journal. 53 (2): 582–88. Bibcode:1989SSASJ..53..582K. doi:10.2136/sssaj1989.03615995005300020047x.
- ^ Lee, Kenneth Ernest & Foster, Ralph C. (2003). "Soil fauna and soil structure". Australian Journal of Soil Research. 29 (6): 745–75. doi:10.1071/SR9910745.
- ^ Scheu, Stefan (2003). "Effects of earthworms on plant growth: patterns and perspectives". Pedobiologia. 47 (5/6): 846–56. doi:10.1078/0031-4056-00270.
- ^ Zhang, Haiquan & Schrader, Stefan (1993). "Earthworm effects on selected physical and chemical properties of soil aggregates". Biology and Fertility of Soils. 15 (3): 229–34. doi:10.1007/BF00361617. S2CID 24151632.
- ^ Bouché, Marcel B. & Al-Addan, Fathel (1997). "Earthworms, water infiltration and soil stability: some new assessments". Soil Biology and Biochemistry. 29 (3/4): 441–52. doi:10.1016/S0038-0717(96)00272-6.
- ^ Bernier, Nicolas (1998). "Earthworm feeding activity and development of the humus profile". Biology and Fertility of Soils. 26 (3): 215–23. doi:10.1007/s003740050370. S2CID 40478203.
- ^ Scheu, Stefan (1991). "Mucus excretion and carbon turnover of endogeic earthworms" (PDF). Biology and Fertility of Soils. 12 (3): 217–20. doi:10.1007/BF00337206. S2CID 21931989. Retrieved 17 December 2017.
- ^ Brown, George G. (1995). "How do earthworms affect microfloral and faunal community diversity?". Plant and Soil. 170 (1): 209–31. doi:10.1007/BF02183068. S2CID 10254688.
- ^ Jouquet, Pascal; Dauber, Jens; Lagerlöf, Jan; Lavelle, Patrick & Lepage, Michel (2006). "Soil invertebrates as ecosystem engineers: intended and accidental effects on soil and feedback loops" (PDF). Applied Soil Ecology. 32 (2): 153–64. doi:10.1016/j.apsoil.2005.07.004. Retrieved 17 December 2017.
- ^ Bohlen, Patrick J.; Scheu, Stefan; Hale, Cindy M.; McLean, Mary Ann; Migge, Sonja; Groffman, Peter M. & Parkinson, Dennis (2004). "Non-native invasive earthworms as agents of change in northern temperate forests" (PDF). Frontiers in Ecology and the Environment. 2 (8): 427–35. doi:10.2307/3868431. JSTOR 3868431. Retrieved 13 August 2017.
- ^ De Bruyn, Lisa Lobry & Conacher, Arthur J. (1990). "The role of termites and ants in soil modification: a review" (PDF). Australian Journal of Soil Research. 28 (1): 55–93. doi:10.1071/SR9900055. Retrieved 17 December 2017.
- ^ Kinlaw, Alton Emory (2006). "Burrows of semi-fossorial vertebrates in upland communities of Central Florida: their architecture, dispersion and ecological consequences" (PDF). pp. 19–45. Retrieved 17 December 2017.
- ^ Borst, George (1968). "The occurrence of crotovinas in some southern California soils" (PDF). Transactions of the 9th International Congress of Soil Science, Adelaide, Australia, August 5–15, 1968. 2. Sidney: Angus & Robertson. pp. 19–27. Retrieved 17 December 2017.
- ^ Gyssels, Gwendolyn; Poesen, Jean; Bochet, Esther & Li, Yong (2005). "Impact of plant roots on the resistance of soils to erosion by water: a review" (PDF). Progress in Physical Geography. 29 (2): 189–217. doi:10.1191/0309133305pp443ra. S2CID 55243167. Retrieved 17 December 2017.
- ^ Balisky, Allen C. & Burton, Philip J. (1993). "Distinction of soil thermal regimes under various experimental vegetation covers". Canadian Journal of Soil Science. 73 (4): 411–20. doi:10.4141/cjss93-043.
- ^ Marrou, Hélène; Dufour, Lydie & Wery, Jacques (2013). "How does a shelter of solar panels influence water flows in a soil-crop system?". European Journal of Agronomy. 50: 38–51. doi:10.1016/j.eja.2013.05.004.
- ^ Heck, Pamela; Lüthi, Daniel & Schär, Christoph (1999). "The influence of vegetation on the summertime evolution of European soil moisture". Physics and Chemistry of the Earth, Part B: Hydrology, Oceans and Atmosphere. 24 (6): 609–14. Bibcode:1999PCEB...24..609H. doi:10.1016/S1464-1909(99)00052-0.
- ^ Jones, David L. (1998). "Organic acids in the rhizospere: a critical review" (PDF). Plant and Soil. 205 (1): 25–44. doi:10.1023/A:1004356007312. S2CID 26813067. Retrieved 17 December 2017.
- ^ Calvaruso, Christophe; Turpault, Marie-Pierre & Frey-Klett, Pascal (2006). "Root-associated bacteria contribute to mineral weathering and to mineral nutrition in trees: a budgeting analysis". Applied and Environmental Microbiology. 72 (2): 1258–66. doi:10.1128/AEM.72.2.1258-1266.2006. PMC 1392890. PMID 16461674.
- ^ Angers, Denis A.; Caron, Jean (1998). "Plant-induced changes in soil structure: processes and feedbacks" (PDF). Biogeochemistry. 42 (1): 55–72. doi:10.1023/A:1005944025343. S2CID 94249645. Retrieved 17 December 2017.
- ^ Dai, Shengpei; Zhang, Bo; Wang, Haijun; Wang, Yamin; Guo, Lingxia; Wang, Xingmei & Li, Dan (2011). "Vegetation cover change and the driving factors over northwest China" (PDF). Journal of Arid Land. 3 (1): 25–33. doi:10.3724/SP.J.1227.2011.00025. Retrieved 17 December 2017.
- ^ Vogiatzakis, Ioannis; Griffiths, Geoffrey H. & Mannion, Antoinette M. (2003). "Environmental factors and vegetation composition, Lefka Ori Massif, Crete, S. Aegean". Global Ecology and Biogeography. 12 (2): 131–46. doi:10.1046/j.1466-822X.2003.00021.x.
- ^ Brêthes, Alain; Brun, Jean-Jacques; Jabiol, Bernard; Ponge, Jean-François & Toutain, François (1995). "Classification of forest humus forms: a French proposal" (PDF). Annales des Sciences Forestières. 52 (6): 535–46. doi:10.1051/forest:19950602. Retrieved 17 December 2017.
- ^ Amundson, Jenny (January 1991). "The Place of Humans in the State Factor Theory of Ecosystems and their Soils". Soil Science: An Interdisciplinary Approach to Soil Research. 151 (1): 99. Bibcode:1991SoilS.151...99A. doi:10.1097/00010694-199101000-00012. S2CID 95061311. Retrieved 30 November 2015.
- ^ Dudal, Rudi (2005). "The sixth factor of soil formation" (PDF). Eurasian Soil Science. 38 (Supplement 1): S60–S65. Retrieved 17 December 2017.
- ^ Anderson, Roger C. (2006). "Evolution and origin of the Central Grassland of North America: climate, fire, and mammalian grazers". Journal of the Torrey Botanical Society. 133 (4): 626–47. doi:10.3159/1095-5674(2006)133[626:EAOOTC]2.0.CO;2.
- ^ Burke, Ingrid C.; Yonker, Caroline M.; Parton, William J.; Cole, C. Vernon; Flach, Klaus & Schimel, David S. (1989). "Texture, climate, and cultivation effects on soil organic matter content in U.S. grassland soils" (PDF). Soil Science Society of America Journal. 53 (3): 800–05. Bibcode:1989SSASJ..53..800B. doi:10.2136/sssaj1989.03615995005300030029x. Retrieved 17 December 2017.
- ^ Lisetskii, Fedor N. & Pichura, Vitalii I. (2016). "Assessment and forecast of soil formation under irrigation in the steppe zone of Ukraine" (PDF). Russian Agricultural Sciences. 42 (2): 155–59. doi:10.3103/S1068367416020075. S2CID 43356998. Retrieved 17 December 2017.
- ^ Schön, Martina (2011). "Impact of N fertilization on subsoil properties: soil organic matter and aggregate stability" (PDF). Retrieved 17 December 2017.
- ^ Jump up to: a b c d Odling-Smee F. J., Laland K. N. & Feldman M. W. (2003). "Niche Construction: The Neglected Process in Evolution (MPB-37)". Princeton University Press. 468 pp. HTM Archived 17 June 2006 at the Wayback Machine, PDF. Chapter 1. page 7-8.
- ^ Ponomarenko, E.V.; Anderson, D.W. (2001), "Importance of charred organic matter in Black Chernozem soils of Saskatchewan", Canadian Journal of Soil Science, 81 (3): 285–297, doi:10.4141/S00-075,
The present paradigm views humus as a system of heteropolycondensates, largely produced by the soil microflora, in varying associations with clay (Anderson 1979). Because this conceptual model, and simulation models rooted within the concept, do not accommodate a large char component, a considerable change in conceptual understanding (a paradigm shift) appears imminent.
- ^ Bormann, Bernard T.; Spaltenstein, Henri; McClellan, Michael H.; Ugolini, Fiorenzo C.; Cromack, Kermit Jr & Nay, Stephan M. (1995). "Rapid soil development after windthrow disturbance in pristine forests" (PDF). Journal of Ecology. 83 (5): 747–57. doi:10.2307/2261411. JSTOR 2261411. Retrieved 17 December 2017.
- ^ Crocker, Robert L. & Major, Jack (1955). "Soil development in relation to vegetation and surface age at Glacier Bay, Alaska" (PDF). Journal of Ecology. 43 (2): 427–48. doi:10.2307/2257005. JSTOR 2257005. Archived from the original (PDF) on 25 September 2017. Retrieved 17 December 2017.
- ^ Jump up to: a b c d Crews, Timothy E.; Kitayama, Kanehiro; Fownes, James H.; Riley, Ralph H.; Herbert, Darrell A.; Mueller-Dombois, Dieter & Vitousek, Peter M. (1995). "Changes in soil phosphorus and ecosystem dynamics along a long term chronosequence in Hawaii" (PDF). Ecology. 76 (5): 1407–24. doi:10.2307/1938144. JSTOR 1938144. Retrieved 17 December 2017.
- ^ Huggett, Richard J. (1998). "Soil chronosequences, soil development, and soil evolution: a critical review". Catena. 32 (3/4): 155–72. doi:10.1016/S0341-8162(98)00053-8.
- ^ Simonson 1957, pp. 20–21.
- ^ Donahue, Miller & Shickluna 1977, p. 26.
- ^ Craft, Christopher; Broome, Stephen & Campbell, Carlton (2002). "Fifteen years of vegetation and soil development after brackish‐water marsh creation" (PDF). Restoration Ecology. 10 (2): 248–58. doi:10.1046/j.1526-100X.2002.01020.x. Archived from the original (PDF) on 10 August 2017. Retrieved 17 December 2017.
- ^ Shipitalo, Martin J. & Le Bayon, Renée-Claire (2004). "Quantifying the effects of earthworms on soil aggregation and porosity" (PDF). In Edwards, Clive A. (ed.). Earthworm ecology (PDF) (2nd ed.). Boca Raton, Florida: CRC Press. pp. 183–200. doi:10.1201/9781420039719.pt5. ISBN 978-1-4200-3971-9. Retrieved 17 December 2017.
- ^ He, Changling; Breuning-Madsen, Henrik & Awadzi, Theodore W. (2007). "Mineralogy of dust deposited during the Harmattan season in Ghana". Geografisk Tidsskrift. 107 (1): 9–15. CiteSeerX 10.1.1.469.8326. doi:10.1080/00167223.2007.10801371. S2CID 128479624.
- ^ Pimentel, David; Harvey, C.; Resosudarmo, Pradnja; Sinclair, K.; Kurz, D.; McNair, M.; Crist, S.; Shpritz, Lisa; Fitton, L.; Saffouri, R. & Blair, R. (1995). "Environmental and economic cost of soil erosion and conservation benefits" (PDF). Science. 267 (5201): 1117–23. Bibcode:1995Sci...267.1117P. doi:10.1126/science.267.5201.1117. PMID 17789193. S2CID 11936877. Archived from the original (PDF) on 13 December 2016. Retrieved 17 December 2017.
- ^ Wakatsuki, Toshiyuki & Rasyidin, Azwar (1992). "Rates of weathering and soil formation" (PDF). Geoderma. 52 (3/4): 251–63. Bibcode:1992Geode..52..251W. doi:10.1016/0016-7061(92)90040-E. Retrieved 17 December 2017.
- ^ Huggett, R.J (1998). "Soil chronosequences, soil development, and soil evolution: a critical review". Catena. 32 (3–4): 155–172. doi:10.1016/S0341-8162(98)00053-8.
- ^ Dokuchaev, V.V., Russian Chernozem
- ^ Jenny, Hans (1980), The Soil Resource - Origin and Behavior, Ecological Studies, 37, New York: Springer-Verlag, ISBN 978-1461261148,
The idea that climate, vegetation, topography, parent material, and time control soils occurs in the writings of early naturalists. An explicit formulation was performed by Dokuchaev in 1898 in an obscure Russian journal unknown to western writers. He set down: soil = f(cl, o, p) tr
- ^ Johnson; et al. (March 2005). "Reflections on the Nature of Soil and Its Biomantle". Annals of the Association of American Geographers. 95: 11–31. doi:10.1111/j.1467-8306.2005.00448.x. S2CID 73651791.
- ^ Pidwirny, M. (2006), Soil Pedogenesis, Fundamentals of Physical Geography (2 ed.)
- ^ C. Michael Hogan. 2008
References[]
| Scholia has a profile for pedogenesis (Q282070). |
- Stanley W. Buol, F.D. Hole and R.W. McCracken. 1997. Soil Genesis and Classification, 4th ed. Iowa State Univ. Press, Ames ISBN 0-8138-2873-2
- C. Michael Hogan. 2008. Makgadikgadi, The Megalithic Portal, ed. A. Burnham [1]
- Francis D. Hole and J.B. Campbell. 1985. Soil landscape analysis. Totowa Rowman & Allanheld, 214 p. ISBN 0-86598-140-X
- Hans Jenny. 1994. Factors of Soil Formation. A System of Quantitative Pedology. New York: Dover Press. (Reprint, with foreword by R. Amundson, of the 1941 McGraw-Hill publication). pdf file format.
- Ben van der Pluijm et al. 2005. Soils, Weathering, and Nutrients from the Global Change 1 Lectures. University of Michigan. Url last accessed on 2007-03-31
- Pedology
- Ecological succession