Sex-determination system

A sex-determination system is a biological system that determines the development of sexual characteristics in an organism. Most organisms that create their offspring using sexual reproduction have two sexes.
In some species there are hermaphrodites.[1] There are also some species that are only one sex due to parthenogenesis, the act of a female reproducing without fertilization.
In many species, sex determination is genetic: males and females have different alleles or even different genes that specify their sexual morphology. In animals this is often accompanied by chromosomal differences, generally through combinations of XY, ZW, XO, ZO chromosomes, or haplodiploidy. The sexual differentiation is generally triggered by a main gene (a "sex locus"), with a multitude of other genes following in a domino effect.
In other cases, sex of a fetus is determined by environmental variables (such as temperature). The details of some sex-determination systems are not yet fully understood. Hopes for future fetal biological system analysis include complete-reproduction-system initialized signals that can be measured during pregnancies to more accurately determine whether a determined sex of a fetus is male, or female. Such analysis of biological systems could also signal whether the fetus is hermaphrodite, which includes total or partial of both male and female reproduction organs.
Some species such as various plants and fish do not have a fixed sex, and instead go through life cycles and change sex based on genetic cues during corresponding life stages of their type. This could be due to environmental factors such as seasons and temperature. In some gonochoric species, a few individuals may have sex characteristics of both sexes, a condition called intersex.[2]
Discovery[]
This section needs expansion. You can help by . (June 2021) |
Sex determination was discovered in the mealworm by the American geneticist Nettie Stevens in 1903.[3][4][5]
Chromosomal systems[]
XX/XY sex chromosomes[]

The XX/XY sex-determination system is the most familiar, as it is found in humans. The XX/XY system is found in most other mammals, as well as some insects. In this system, most females have two of the same kind of sex chromosome (XX), while most males have two distinct sex chromosomes (XY). The X and Y sex chromosomes are different in shape and size from each other, unlike the rest of the chromosomes (autosomes), and are sometimes called allosomes. In some species, such as humans, organisms remain sex indifferent for a time after they're created; in others, however, such as fruit flies, sexual differentiation occurs as soon as the egg is fertilized.[6]
Y-centered sex determination[]
Some species (including humans) have a gene SRY on the Y chromosome that determines maleness. Members of SRY-reliant species can have uncommon XY chromosomal combinations such as XXY and still live.[6] Human sex is determined by the presence or absence of a Y chromosome with a functional SRY gene. Once the SRY gene is activated, cells create testosterone and anti-müllerian hormone which typically ensures the development of a single, male reproductive system.[6] In typical XX embryos, cells secrete estrogen, which drives the body toward the female pathway.
In Y-centered sex determination, the SRY gene is the main gene in determining male characteristics, but multiple genes are required to develop testes. In XY mice, lack of the gene DAX1 on the X chromosome results in sterility, but in humans it causes adrenal hypoplasia congenita.[7] However, when an extra DAX1 gene is placed on the X chromosome, the result is a female, despite the existence of SRY.[8] Even when there are normal sex chromosomes in XX females, duplication or expression of SOX9 causes testes to develop.[9][10] Gradual sex reversal in developed mice can also occur when the gene FOXL2 is removed from females.[11] Even though the gene DMRT1 is used by birds as their sex locus, species who have XY chromosomes also rely upon DMRT1, contained on chromosome 9, for sexual differentiation at some point in their formation.[6]
X-centered sex determination[]
Some species, such as fruit flies, use the presence of two X chromosomes to determine femaleness.[12] Species that use the number of Xs to determine sex are nonviable with an extra X chromosome.
Other variants of XX/XY sex determination[]
Some fish have variants of the XY sex-determination system, as well as the regular system. For example, while having an XY format, Xiphophorus nezahualcoyotl and X. milleri also have a second Y chromosome, known as Y', that creates XY' females and YY' males.[13]
At least one monotreme, the platypus, presents a particular sex determination scheme that in some ways resembles that of the ZW sex chromosomes of birds and lacks the SRY gene. The platypus has ten sex chromosomes; males have an XYXYXYXYXY pattern while females have ten X chromosomes. Although it is an XY system, the platypus' sex chromosomes share no homologues with eutherian sex chromosomes.[14] Instead, homologues with eutherian sex chromosomes lie on the platypus chromosome 6, which means that the eutherian sex chromosomes were autosomes at the time that the monotremes diverged from the therian mammals (marsupials and eutherian mammals). However, homologues to the avian DMRT1 gene on platypus sex chromosomes X3 and X5 suggest that it is possible the sex-determining gene for the platypus is the same one that is involved in bird sex-determination. More research must be conducted in order to determine the exact sex determining gene of the platypus.[15]

XX/X0 sex chromosomes[]
In this variant of the XY system, females have two copies of the sex chromosome (XX) but males have only one (X0). The 0 denotes the absence of a second sex chromosome. Generally in this method, the sex is determined by amount of genes expressed across the two chromosomes. This system is observed in a number of insects, including the grasshoppers and crickets of order Orthoptera and in cockroaches (order Blattodea). A small number of mammals also lack a Y chromosome. These include the Amami spiny rat (Tokudaia osimensis) and the Tokunoshima spiny rat (Tokudaia tokunoshimensis) and Sorex araneus, a shrew species. Transcaucasian mole voles (Ellobius lutescens) also have a form of XO determination, in which both sexes lack a second sex chromosome.[8] The mechanism of sex determination is not yet understood.[16]
The nematode C. elegans is male with one sex chromosome (X0); with a pair of chromosomes (XX) it is a hermaphrodite.[17] Its main sex gene is XOL, which encodes XOL-1 and also controls the expression of the genes TRA-2 and HER-1. These genes reduce male gene activation and increase it, respectively.[18]
ZW/ZZ sex chromosomes[]
The ZW sex-determination system is found in birds, some reptiles, and some insects and other organisms. The ZW sex-determination system is reversed compared to the XY system: females have two different kinds of chromosomes (ZW), and males have two of the same kind of chromosomes (ZZ). In the chicken, this was found to be dependent on the expression of DMRT1.[19] In birds, the genes FET1 and ASW are found on the W chromosome for females, similar to how the Y chromosome contains SRY.[6] However, not all species depend upon the W for their sex. For example, there are moths and butterflies that are ZW, but some have been found female with ZO, as well as female with ZZW.[17] Also, while mammals deactivate one of their extra X chromosomes when female, it appears that in the case of Lepidoptera, the males produce double the normal amount of enzymes, due to having two Z's.[17] Because the use of ZW sex determination is varied, it is still unknown how exactly most species determine their sex.[17] However, reportedly, the silkworm Bombyx mori uses a single female-specific piRNA as the primary determiner of sex.[20] Despite the similarities between the ZW and XY systems, these sex chromosomes evolved separately. In the case of the chicken, their Z chromosome is more similar to humans' autosome 9.[21] The chicken's Z chromosome also seems to be related to the X chromosome of the platypus.[22] When a ZW species, such as the Komodo dragon, reproduces parthenogenetically, usually only males are produced. This is due to the fact that the haploid eggs double their chromosomes, resulting in ZZ or WW. The ZZ become males, but the WW are not viable and are not brought to term.[23]
In both XY and ZW sex determination systems, the sex chromosome carrying the critical factors is often significantly smaller, carrying little more than the genes necessary for triggering the development of a given sex.[24][better source needed]
ZZ/Z0 sex chromosomes[]
The ZZ/Z0 sex-determination system is found in some moths. In these insects there is one sex chromosome, Z. Males have two Z chromosomes, whereas females have one Z. Males are ZZ, while females are Z0.[25][26][27]
UV sex chromosomes[]
In some Bryophyte and some algae species, the gametophyte stage of the life cycle, rather than being hermaphrodite, occurs as separate male or female individuals that produce male and female gametes respectively. When meiosis occurs in the sporophyte generation of the life cycle, the sex chromosomes known as U and V assort in spores that carry either the U chromosome and give rise to female gametophytes, or the V chromosome and give rise to male gametophytes.[28][29]
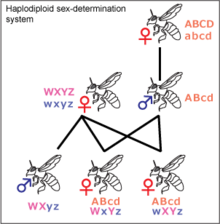
Haplodiploidy[]
Haplodiploidy is found in insects belonging to Hymenoptera, such as ants and bees. Sex determination is controlled by the zygosity of a complementary sex determiner (csd) locus. Unfertilized eggs develop into haploid individuals which have a single, hemizygous copy of the csd locus and are therefore males. Fertilized eggs develop into diploid individuals which, due to high variability in the csd locus, are generally heterozygous females. In rare instances diploid individuals may be homozygous, these develop into sterile males. The gene acting as a csd locus has been identified in the honeybee and several candidate genes have been proposed as a csd locus for other Hymenopterans.[30][31][32] Most females in the Hymenoptera order can decide the sex of their offspring by holding received sperm in their spermatheca and either releasing it into their oviduct or not. This allows them to create more workers, depending on the status of the colony.[33]
Environmental systems[]

Temperature-dependent[]
Many other sex-determination systems exist. In some species of reptiles, including alligators, some turtles, and the tuatara, sex is determined by the temperature at which the egg is incubated during a temperature-sensitive period. There are no examples of temperature-dependent sex determination (TSD) in birds. Megapodes had formerly been thought to exhibit this phenomenon, but were found to actually have different temperature-dependent embryo mortality rates for each sex.[34] For some species with TSD, sex determination is achieved by exposure to hotter temperatures resulting in the offspring being one sex and cooler temperatures resulting in the other. This type of TSD is called Pattern I. For others species using TSD, it is exposure to temperatures on both extremes that results in offspring of one sex, and exposure to moderate temperatures that results in offspring of the opposite sex, called Pattern II TSD. The specific temperatures required to produce each sex are known as the female-promoting temperature and the male-promoting temperature.[35] When the temperature stays near the threshold during the temperature sensitive period, the sex ratio is varied between the two sexes.[36] Some species' temperature standards are based on when a particular enzyme is created. These species that rely upon temperature for their sex determination do not have the SRY gene, but have other genes such as DAX1, DMRT1, and SOX9 that are expressed or not expressed depending on the temperature.[35] The sex of some species, such as the Nile tilapia, Australian skink lizard, and Australian dragon lizard, is initially determined by chromosomes, but can later be changed by the temperature of incubation.[13]
It is unknown how exactly temperature-dependent sex determination evolved.[37] It could have evolved through certain sexes being more suited to certain areas that fit the temperature requirements. For example, a warmer area could be more suitable for nesting, so more females are produced to increase the amount that nest next season.[37] Environmental sex determination preceded the genetically determined systems of birds and mammals; it is thought that a temperature-dependent amniote was the common ancestor of amniotes with sex chromosomes.[38]
Other systems[]
There are other environmental sex determination systems including location-dependent determination systems as seen in the marine worm Bonellia viridis – larvae become males if they make physical contact with a female, and females if they end up on the bare sea floor. This is triggered by the presence of a chemical produced by the females, bonellin.[39] Some species, such as some snails, practice sex change: adults start out male, then become female. In tropical clown fish, the dominant individual in a group becomes female while the other ones are male, and bluehead wrasses (Thalassoma bifasciatum) are the reverse. Some species, however, have no sex-determination system. Hermaphrodite species include the common earthworm and certain species of snails. A few species of fish, reptiles, and insects reproduce by parthenogenesis and are female altogether. There are some reptiles, such as the boa constrictor and Komodo dragon that can reproduce both sexually and asexually, depending on whether a mate is available.[40]
Other unusual systems include those of the green swordtail (a polyfactorial system with the sex-determining genes on several chromosomes)[13] the Chironomus midges[clarification needed][citation needed]; the of zebrafish, with an unknown trigger;[13] and the platyfish, which has W, X, and Y chromosomes. This allows WY, WX, or XX females and YY or XY males.[13]
Evolution[]
Origin of sex chromosomes[]
Chromosomal sex determination may have evolved early in the history of eukaryotes.[41] The accepted hypothesis of XY and ZW sex chromosome evolution is that they evolved at the same time, in two different branches.[42][43]
No genes are shared between the avian ZW and mammal XY chromosomes[44] and the chicken Z chromosome is similar to the human autosomal chromosome 9, rather than X or Y. This suggests not that the ZW and XY sex-determination systems share an origin but that the sex chromosomes are derived from autosomal chromosomes of the common ancestor of birds and mammals. In the platypus, a monotreme, the X1 chromosome shares homology with therian mammals, while the X5 chromosome contains an avian sex-determination gene, further suggesting an evolutionary link.[45]
However, there is some evidence to suggest that there could have been transitions between ZW and XY, such as in Xiphophorus maculatus, which have both ZW and XY systems in the same population, despite the fact that ZW and XY have different gene locations.[46][47] A recent theoretical model raises the possibility of both transitions between the XY/XX and ZZ/ZW system and environmental sex determination[48] The platypus' genes also back up the possible evolutionary link between XY and ZW, because they have the DMRT1 gene possessed by birds on their X chromosomes.[49] Regardless, XY and ZW follow a similar route. All sex chromosomes started out as an original autosome of an original amniote that relied upon temperature to determine the sex of offspring. After the mammals separated, the branch further split into Lepidosauria and Archosauromorpha. These two groups both evolved the ZW system separately, as evidenced by the existence of different sex chromosomal locations.[43] In mammals, one of the autosome pair, now Y, mutated its SOX3 gene into the SRY gene, causing that chromosome to designate sex.[43][49][50] After this mutation, the SRY-containing chromosome inverted and was no longer completely homologous with its partner. The regions of the X and Y chromosomes that are still homologous to one another are known as the pseudoautosomal region.[51] Once it inverted, the Y chromosome became unable to remedy deleterious mutations, and thus degenerated.[43] There is some concern that the Y chromosome will shrink further and stop functioning in ten million years: but the Y chromosome has been strictly conserved after its initial rapid gene loss.[52][53]
There are some species, such as the medaka fish, that evolved sex chromosomes separately; their Y chromosome never inverted and can still swap genes with the X. These species' sex chromosomes are relatively primitive and unspecialized. Because the Y does not have male-specific genes and can interact with the X, XY and YY females can be formed as well as XX males.[13] Non-inverted Y chromosomes with long histories are found in pythons and emus, each system being more than 120 million years old, suggesting that inversions are not necessarily an eventuality.[54]
XO sex determination can evolve from XY sex determination with about 2 million years.[55]
In plants it has been suggested that sex chromosomes evolved less than 20 million years ago.[56]
See also[]
- Clarence Erwin McClung, who discovered the role of chromosomes in sex determination
- Testis-determining factor
- Maternal influence on sex determination
- Sequential hermaphroditism
- Sex determination and differentiation (human)
- Tetrahymena have seven sexes
- Schizophyllum commune have 23,328 sexes
References[]
- ^ Rosenfield, Kevin A. (2018), Vonk, Jennifer; Shackelford, Todd (eds.), "Hermaphrodite", Encyclopedia of Animal Cognition and Behavior, Cham: Springer International Publishing, pp. 1–2, doi:10.1007/978-3-319-47829-6_329-1, ISBN 978-3-319-47829-6, retrieved 4 May 2021
- ^ Minelli A, Fusco G. "The Biology of Reproduction". Cambridge University Press. pp. 116–117. Archived from the original on 11 October 2020. Retrieved 11 October 2020.
- ^ "Nettie Stevens: A Discoverer of Sex Chromosomes | Learn Science at Scitable". www.nature.com. Retrieved 7 June 2018.
- ^ Ogilvie, Marilyn Bailey; Choquette, Clifford J. (1981). "Nettie Maria Stevens (1861–1912): Her Life and Contributions to Cytogenetics". Proceedings of the American Philosophical Society. 125 (4): 292–311. JSTOR 986332. PMID 11620765.
- ^ "Nettie Maria Stevens (1861–1912) | The Embryo Project Encyclopedia". embryo.asu.edu. Retrieved 7 June 2018.
- ^ Jump up to: a b c d e Hake, Laura (2008). "Genetic Mechanisms of Sex Determination". Nature Education. 1 (1). Retrieved 8 December 2011.
- ^ Goodfellow, P. N.; Camerino, G. (1999). "DAX-1, an 'antitestis' gene". Cellular and Molecular Life Sciences. 55 (6–7): 857–863. doi:10.1007/PL00013201. PMID 10412368. S2CID 19764423.
- ^ Jump up to: a b Chandra, H. S. (25 April 1999). "Another way of looking at the enigma of sex determination in Ellobius lutescens". Current Science. 76 (8): 1072.
- ^ Cox, James J.; Willatt, L; Homfray, T; Woods, C. G. (6 January 2011). "A SOX9 Duplication and Familial 46,XX Developmental Testicular Disorder". New England Journal of Medicine. 364 (1): 91–93. doi:10.1056/NEJMc1010311. PMID 21208124.
- ^ Huang, Bing; Wang, S; Ning, Y; Lamb, A. N.; Bartley, J . (7 December 1999). "Autosomal XX sex reversal caused by duplication of SOX9". American Journal of Medical Genetics. 87 (4): 349–353. doi:10.1002/(SICI)1096-8628(19991203)87:4<349::AID-AJMG13>3.0.CO;2-N. PMID 10588843.
- ^ Uhlenhaut, Henriette N.; Jakob, S; Anlag, K; Eisenberger, T; Sekido, R; Kress, J; Treier, A. C.; Klugmann, C; Klasen, C; Holter, N. I.; Riethmacher, D; Schütz, G; Cooney, A. J.; Lovell-Badge, R; Treier, M (11 December 2009). "Somatic Sex Reprogramming of Adult Ovaries to Testes by FOXL2 Ablation". Cell. 139 (6): 1130–1142. doi:10.1016/j.cell.2009.11.021. PMID 20005806.
- ^ Penalva, Luiz O. F.; Sánchez (September 2003). "RNA Binding Protein Sex-Lethal (Sxl) and Control of Drosophila Sex Determination and Dosage Compensation". Microbiology and Molecular Biology Reviews. 67 (3): 343–359. doi:10.1128/MMBR.67.3.343-359.2003. PMC 193869. PMID 12966139.
- ^ Jump up to: a b c d e f Schartl, Manfred (July 2004). "A comparative view on sex determination in medaka". Mechanisms of Development. 121 (7–8): 639–645. doi:10.1016/j.mod.2004.03.001. PMID 15210173. S2CID 17401686.
- ^ Warren, W.C.; Hillier, Ladeana W.; Marshall Graves, Jennifer A.; Birney, Ewan; Ponting, Chris P.; Grützner, Frank; Belov, Katherine; Miller, Webb; et al. (2008). "Genome analysis of the platypus reveals unique signatures of evolution". Nature. 453 (7192): 175–U1. Bibcode:2008Natur.453..175W. doi:10.1038/nature06936. PMC 2803040. PMID 18464734.
- ^ Gruetzner, F.; T. Ashley; D. M. Rowell & J. A. M. Graves (2006). "Analysis of the platypus reveals unique signatures of evolution". Chromosoma. 115 (2): 75–88. doi:10.1007/s00412-005-0034-4. PMID 16344965. S2CID 23603889.
- ^ Kuroiwa A, Handa S, Nishiyama C, Chiba E, Yamada F, Abe S, Matsuda Y (8 June 2011). "Additional copies of CBX2 in the genomes of males of mammals lacking SRY, the Amami spiny rat (Tokudaia osimensis) and the Tokunoshima spiny rat (Tokudaia tokunoshimensis)". Chromosome Res. 19 (5): 635–44. doi:10.1007/s10577-011-9223-6. PMID 21656076. S2CID 23311263.
- ^ Jump up to: a b c d (Majerus 2003, p. 60)
- ^ Patricia E. Kuwabara; Peter G. Okkema; Judith Kimble (April 1992). "tra-2 Encodes a Membrane Protein and May Mediate Cell Communication in the Caenorhabditis elegans Sex Determination Pathway". Molecular Biology of the Cell. 3 (4): 461–73. doi:10.1091/mbc.3.4.461. PMC 275596. PMID 1498366.
- ^ Smith, C. A.; Roeszler, K. N.; Ohnesorg, T.; Cummins, D. M.; Farlie, P. G.; Doran, T. J.; Sinclair, A. H. (September 2009). "The avian Z-linked gene DMRT1 is required for male sex determination in the chicken". Nature. 461 (7261): 267–271. Bibcode:2009Natur.461..267S. doi:10.1038/nature08298. PMID 19710650. S2CID 4413389.
- ^ Kiuchi, Takashi; Koga, Hikaru; Kawamoto, Munetaka; Shoji, Keisuke; Sakai, Hiroki; Arai, Yuji; Ishihara, Genki; Kawaoka, Shinpei; Sugano, Sumio; Shimada, Toru; Suzuki, Yutaka; Suzuki, Masataka; Katsuma, Susumu (14 May 2014). "A single female-specific piRNA is the primary determiner of sex in the silkworm". Nature. 509 (7502): 633–636. Bibcode:2014Natur.509..633K. doi:10.1038/nature13315. PMID 24828047. S2CID 205238635.
- ^ Stiglec, R.; Ezaz, T.; Graves, J. A. (2007). "A new look at the evolution of avian sex chromosomes". Cytogenet. Genome Res. 117 (1–4): 103–9. doi:10.1159/000103170. PMID 17675850. S2CID 12932564.
- ^ Grützner, F.; Rens, W.; Tsend-Ayush, E.; El-Mogharbel, N.; O'Brien, P. C. M.; Jones, R. C.; Ferguson-Smith, M. A. & Marshall, J. A. (2004). "In the platypus a meiotic chain of ten sex chromosomes shares genes with the bird Z and mammal X chromosomes". Nature. 432 (7019): 913–917. Bibcode:2004Natur.432..913G. doi:10.1038/nature03021. PMID 15502814. S2CID 4379897.
- ^ "Virgin births for giant lizards". BBC News. 20 December 2006. Retrieved 13 March 2008.
- ^ "Evolution of the Y Chromosome". Annenberg Media. Annenberg Media. Archived from the original on 4 November 2004. Retrieved 1 April 2008.
- ^ Traut, W.; Sahara, K.; Marec, F. (2007). "Sex Chromosomes and Sex Determination in Lepidoptera". Sexual Development. 1 (6): 332–346. doi:10.1159/000111765. PMID 18391545. S2CID 6885122.
- ^ "Genetic Mechanisms of Sex Determination - Learn Science at Scitable". www.nature.com.
- ^ Handbuch Der Zoologie / Handbook of Zoology. Walter de Gruyter. 1925. ISBN 9783110162103 – via Google Books.
- ^ Bachtrog D, Kirkpatrick M, Mank JE, McDaniel SF, Pires JC, Rice W, Valenzuela N (September 2011). "Are all sex chromosomes created equal?". Trends Genet. 27 (9): 350–7. doi:10.1016/j.tig.2011.05.005. PMID 21962970.
- ^ Renner, S. S.; Heinrichs, J.; Sousa, A. (2017). "The sex chromosomes of bryophytes: Recent insights, open questions, and reinvestigations of Frullania dilatata and Plagiochila asplenioides". Journal of Systematics and Evolution. 55 (4): 333–339. doi:10.1111/jse.12266.
- ^ Beye, Martin; Hasselmann, Martin; Fondrk, M.Kim; Page, Robert E; Omholt, Stig W (August 2003). "The Gene csd Is the Primary Signal for Sexual Development in the Honeybee and Encodes an SR-Type Protein". Cell. 114 (4): 419–429. doi:10.1016/S0092-8674(03)00606-8.
- ^ Privman, Eyal; Wurm, Yannick; Keller, Laurent (7 May 2013). "Duplication and concerted evolution in a master sex determiner under balancing selection". Proceedings of the Royal Society B: Biological Sciences. 280 (1758): 20122968. doi:10.1098/rspb.2012.2968.
- ^ Miyakawa, Misato Okamoto; Tsuchida, Koji; Miyakawa, Hitoshi (March 2018). "The doublesex gene integrates multi-locus complementary sex determination signals in the Japanese ant, Vollenhovia emeryi". Insect Biochemistry and Molecular Biology. 94: 42–49. doi:10.1016/j.ibmb.2018.01.006.
- ^ van Wilgenburg, Ellen; Driessen, Gerard; Beukeboom, Leow (5 January 2006). "Single locus complementary sex determination in Hymenoptera: An "unintelligent" design?". Frontiers in Zoology. 3 (1): 1. doi:10.1186/1742-9994-3-1. PMC 1360072. PMID 16393347.
- ^ Göth, Ann; Booth, David T. (22 March 2005). "Temperature-dependent sex ratio in a bird". Biology Letters. 1 (1): 31–33. doi:10.1098/rsbl.2004.0247. PMC 1629050. PMID 17148121.
- ^ Jump up to: a b Torres Maldonado LC, Landa Piedra A, Moreno Mendoza N, Marmolejo Valencia A, Meza Martínez A, Merchant Larios H (October 2002). "Expression profiles of Dax1, Dmrt1, and Sox9 during temperature sex determination in gonads of the sea turtle Lepidochelys olivacea". Gen. Comp. Endocrinol. 129 (1): 20–6. doi:10.1016/s0016-6480(02)00511-7. PMID 12409092.
- ^ Bull, J. J. (March 1980). "Sex Determination in Reptiles". The Quarterly Review of Biology. 55 (1): 3–21. doi:10.1086/411613. JSTOR 2826077. S2CID 85177125.
- ^ Jump up to: a b Valenzuela, Nicole; Janzen, Fredric J. (2001). "Nest-site philopatry and the evolution of temperature-dependent sex determination" (PDF). Evolutionary Ecology Research. 3: 779–794. Retrieved 7 December 2011.
- ^ Janzen, F. J.; Phillips, P. C. (12 May 2006). "Exploring the evolution of environmental sex determination, especially in reptiles". Journal of Evolutionary Biology. 19 (6): 1775–1784. doi:10.1111/j.1420-9101.2006.01138.x. PMID 17040374. S2CID 15485510.
- ^ Gilbert, Scott (2006). Developmental biology (8th. ed.). Sunderland, Mass.: Sinauer Associates, Inc. Publishers. pp. 550–553. ISBN 9780878932504.
- ^ Watts, Phillip C.; Buley, Kevin R.; Sanderson, Stephanie; Boardman, Wayne; Ciofi, Claudio & Gibson, Richard (21 December 2006). "Parthenogenesis in Komodo dragons". Nature. 444 (7122): 1021–1022. Bibcode:2006Natur.444.1021W. doi:10.1038/4441021a. PMID 17183308. S2CID 4311088.
- ^ Lehtonen J, Parker GA (2014). "Gamete competition, gamete limitation, and the evolution of the two sexes". Molecular Human Reproduction. 20 (12): 1161–1168. doi:10.1093/molehr/gau068. PMID 25323972.
- ^ Namekawa, Satoshi; Lee, Jeannie T. (2009). "XY and ZW: Is Meiotic Sex Chromosome Inactivation the Rule in Evolution?". PLOS Genetics. 5 (5): 3. doi:10.1371/journal.pgen.1000493. PMC 2679206. PMID 19461890.
- ^ Jump up to: a b c d Vallender, Eric; Lahn, B. T. (28 November 2006). "Multiple independent origins of sex chromosomes in amniotes". Proceedings of the National Academy of Sciences. 103 (5): 18031–2. Bibcode:2006PNAS..10318031V. doi:10.1073/pnas.0608879103. PMC 1838700. PMID 17116892.
- ^ Stiglec R, Ezaz T, Graves JA (2007). "A new look at the evolution of avian sex chromosomes". Cytogenetic and Genome Research. 117 (1–4): 103–9. doi:10.1159/000103170. PMID 17675850. S2CID 12932564.
- ^ Veyrunes F, Waters PD, Miethke P, Rens W, McMillan D, Alsop AE, et al. (June 2008). "Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes". Genome Research. 18 (6): 965–73. doi:10.1101/gr.7101908. PMC 2413164. PMID 18463302.
- ^ Graves, Jennifer (1 September 2000). "Human Y Chromosome, Sex Determination, and Spermatogenesis—A Feminist View". Biology of Reproduction. 63 (3): 667–676. doi:10.1095/biolreprod63.3.667b. PMID 10952906.
- ^ Ezaz, Tariq; Stiglec, Rami; Veyrunes, Frederic; Marshall Graves, Jennifer A. (5 September 2006). "Relationships between Vertebrate ZW and XY Sex Chromosome System". Current Biology. 16 (17): R736–43. doi:10.1016/j.cub.2006.08.021. hdl:1885/37887. PMID 16950100. S2CID 18864471.
- ^ Quinn, A. E.; Stephen D. Sarre; Jennifer A. Marshall Graves; Arthur Georges; Georges, A. (6 January 2011). "Evolutionary transitions between mechanisms of sex determination in vertebrates". Biology Letters. 7 (3): 443–8. doi:10.1098/rsbl.2010.1126. PMC 3097877. PMID 21212104.
- ^ Jump up to: a b Graves, Jennifer (10 March 2006). "Sex Chromosome Specialization and Degeneration in Mammals". Cell. 124 (5): 901–914. doi:10.1016/j.cell.2006.02.024. PMID 16530039. S2CID 8379688.
- ^ "The evolution of the sex chromosomes: Step by step" (Press release). University of Chicago Medical Center. 28 October 1999. Retrieved 23 October 2011.
- ^ Charlesworth, Brian (14 August 2003). "The organization and evolution of the human Y chromosome". Genome Biology. 4 (9): 226. doi:10.1186/gb-2003-4-9-226. PMC 193647. PMID 12952526.
- ^ Graves, Jennifer (22 July 2004). "The degenerate Y chromosome – can conversion save it?". Reproduction, Fertility, and Development. 16 (5): 527–34. doi:10.1071/RD03096. PMID 15367368. S2CID 23740483.
- ^ Hughes JF, et al. (22 February 2012). "Strict evolutionary conservation followed rapid gene loss on human and rhesus Y chromosomes". Nature. 483 (7387): 82–86. Bibcode:2012Natur.483...82H. doi:10.1038/nature10843. PMC 3292678. PMID 22367542.
- ^ Bachtrog D, Mank JE, Peichel CL, Kirkpatrick M, Otto SP, Ashman TL, Hahn MW, Kitano J, Mayrose I, Ming R, Perrin N, Ross L, Valenzuela N, Vamosi JC, Tree of Sex Consortium (July 2014). "Sex determination: why so many ways of doing it?". PLOS Biology. 12 (7): e1001899. doi:10.1371/journal.pbio.1001899. PMC 4077654. PMID 24983465.
- ^ Nei, Masatoshi (2 May 2013). Mutation-Driven Evolution. OUP Oxford. p. 168. ISBN 978-0-19-163781-0.
- ^ Clarke, Robert; Merlin, Mark (28 June 2016). Cannabis: Evolution and Ethnobotany. Univ of California Press. p. 359. ISBN 978-0-520-29248-2.
Bibliography[]
- Majerus, M. E. N. (2003). Sex wars: genes, bacteria, and biased sex ratios. Princeton University Press. p. 250. ISBN 978-0-691-00981-0. Retrieved 4 November 2011.
- Beukeboom, L. & Perrin, N. (2014). The Evolution of Sex Determination. Oxford University Press. Online resources.
- Sex-determination systems
- Epigenetics