Small-cell carcinoma
| Small-cell carcinoma | |
|---|---|
| Other names | Small-cell lung cancer, Oat-cell carcinoma |
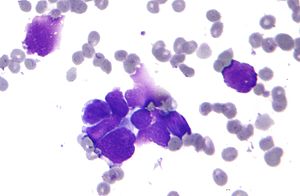 | |
| Micrograph of a small-cell carcinoma of the lung showing cells with nuclear moulding, minimal amount of cytoplasm and stippled chromatin. FNA specimen. Field stain. | |
| Specialty | Oncology |
Small-cell carcinoma is a type of highly malignant cancer that most commonly arises within the lung,[1] although it can occasionally arise in other body sites, such as the cervix,[2] prostate,[3] and gastrointestinal tract. Compared to non-small cell carcinoma, small cell carcinoma has a shorter doubling time, higher growth fraction, and earlier development of metastases.
Extensive stage small cell lung cancer is classified as a rare disorder.[4] Ten-year relative survival rate is 3.5%; however, women have a higher survival rate, 4.3%, and men lower, 2.8%.[5] Survival can be higher or lower based on a combination of factors including stage, age, gender and race.[6]
Types of SCLC[]
Small-cell lung carcinoma has long been divided into two clinicopathological stages, termed limited stage (LS) and extensive stage (ES).[7] The stage is generally determined by the presence or absence of metastases, whether or not the tumor appears limited to the thorax, and whether or not the entire tumor burden within the chest can feasibly be encompassed within a single radiotherapy portal.[8] In general, if the tumor is confined to one lung and the lymph nodes close to that lung, the cancer is said to be LS. If cancer has spread beyond that, it is said to be ES.
LS-SCLC[]
In cases of LS-SCLC, combination chemotherapy (usually cisplatin or carboplatin plus etoposide) is administered together with concurrent chest radiotherapy (RT).[9][10][11]
Chest RT has been shown to improve survival in LS-SCLC.[12]
Exceptionally high objective initial response rates (RR) of between 60% and 90% are seen in LS-SCLC using chemotherapy alone, with between 45% and 75% of individuals showing a "complete response" (CR), which is defined as the disappearance of all radiological and clinical signs of tumor. However, relapse rate remains high, and median survival is only 18 to 24 months.[citation needed]
Because SCLC usually metastasizes widely very early on in the natural history of the tumor, and because nearly all cases respond dramatically to chemotherapy and/or radiotherapy, there has been little role for surgery in this disease since the 1970s.[13] However, recent work suggests that in cases of small, asymptomatic, node-negative SCLC's ("very limited stage"), surgical excision may improve survival when used prior to chemotherapy ("adjuvant chemotherapy").[14]
ES-SCLC[]
In ES-SCLC, platinum-based combination chemotherapy is the standard of care,[15] with radiotherapy added only to palliate symptoms such as dyspnea, pain from liver or bone metastases, or for treatment of brain metastases, which, in small-cell lung carcinoma, typically have a rapid, if temporary, response to whole brain radiotherapy.[citation needed]
Combination chemotherapy consists of a wide variety of agents, including cisplatin, cyclophosphamide, vincristine and carboplatin. Response rates are high even in extensive disease, with between 15% and 30% of subjects having a complete response to combination chemotherapy, and the vast majority having at least some objective response. Responses in ES-SCLC are often of short duration, and the evidence surrounding the risk of treatment compared to the potential benefit of chemotherapy for people who have extensive SCLC is not clear.[15]
If complete response to chemotherapy occurs in a subject with SCLC, then prophylactic cranial irradiation (PCI) is often used in an attempt to prevent the emergence of brain metastases. Although this treatment is often effective, it can cause hair loss and fatigue. Prospective randomized trials with almost two years of follow-up have not shown neurocognitive ill-effects. Meta-analyses of randomized trials confirm that PCI provides significant survival benefits.[citation needed]
In August 2018, the FDA approved nivolumab to treat patients with metastatic small cell lung cancer (SCLC) who failed to respond to platinum-based chemotherapy and at least one other line of treatment. Nivolumab is approved in more than 60 countries. According to LUNGevity Foundation, “This approval marks a major milestone for the patients touched by this unrelenting disease and may motivate them to pursue further treatment where there previously were no other approved options.”[16]
In September 2018, the results from the global, randomized phase I/III IMpower 133 trial were announced at the World Congress on Lung Cancer in Toronto, ON. In this study, patients with ES-SCLC were treated with standard carboplatin plus etoposide and were randomized to receive atezolizumab or placebo. Atezolizumab was associated with a significant improvement in overall survival (HR for death = 0.70)[17]
Signs and symptoms[]

Small-cell carcinoma of the lung usually presents in the central airways and infiltrates the submucosa leading to narrowing of bronchial airways. Common symptoms include cough, dyspnea, weight loss, and debility. Over 70% of patients with small-cell carcinoma present with metastatic disease; common sites include liver, adrenals, bone, and brain.[citation needed]
Due to its high grade neuroendocrine nature, small-cell carcinomas can produce ectopic hormones, including adrenocorticotropic hormone (ACTH) and anti-diuretic hormone (ADH). Ectopic production of large amounts of ADH leads to syndrome of inappropriate antidiuretic hormone hypersecretion (SIADH).[18] Lambert-Eaton myasthenic syndrome (LEMS) is a well-known paraneoplastic condition linked to small-cell carcinoma.[19]
Small cell lung cancer[]

When associated with the lung, it is sometimes called "oat cell carcinoma" due to the flat cell shape and scanty cytoplasm. Caution is required when diagnosing SCLC because small cell mesothelioma – an extremely rare subtype of lung cancer – can be mistaken for small cell lung cancer.[20]
It is thought to originate from neuroendocrine cells (APUD cells) in the bronchus called Feyrter cells (named for Friedrich Feyrter).[21] Hence, they express a variety of neuroendocrine markers, and may lead to ectopic production of hormones like ADH and ACTH that may result in paraneoplastic syndromes and Cushing's syndrome.[22] Approximately half of all individuals diagnosed with Lambert-Eaton myasthenic syndrome (LEMS) will eventually be found to have a small-cell carcinoma of the lung.[19]
Small-cell carcinoma is most often more rapidly and widely metastatic than non-small-cell lung carcinoma[23] (and hence staged differently). There is usually early involvement of the hilar and mediastinal lymph nodes. [22] The mechanisms of its metastatic progression are not well-understood.[24]
Combined small-cell lung carcinoma (c-SCLC)[]
Small-cell lung carcinoma can occur in combination with a wide variety of other histological variants of lung cancer,[25] including extremely complex malignant tissue admixtures.[26] [27] When it is found with one or more differentiated forms of lung cancer, such as squamous cell carcinoma or adenocarcinoma, the malignant tumor is then diagnosed and classified as a combined small cell lung carcinoma (c-SCLC).[25] C-SCLC is the only currently recognized subtype of SCLC.[25]
Although combined small-cell lung carcinoma is currently staged and treated similarly to "pure" small-cell carcinoma of the lung, recent research suggests surgery might improve outcomes in very early stages of this tumor type.[citation needed]
Smoking is a significant risk factor. Symptoms and signs are as for other lung cancers. In addition, because of their neuroendocrine cell origin, small-cell carcinomas will often secrete substances that result in paraneoplastic syndromes such as Lambert-Eaton myasthenic syndrome.[28]
Extrapulmonary small-cell carcinoma[]
Very rarely, the primary site for small-cell carcinoma is outside of the lungs and pleural space; in these cases, it is referred to as extrapulmonary small-cell carcinoma (EPSCC). Outside of the respiratory tract, small-cell carcinoma can appear in the cervix, prostate, liver, pancreas, gastrointestinal tract, or bladder.[29] It is estimated to account for 1,000 new cases a year in the U.S. Histologically similar to small-cell lung cancer, therapies for small-cell lung cancer are usually used to treat EPSCC.[30] First-line treatment is usually with cisplatin and etoposide. In Japan, first-line treatment is shifting to irinotecan and cisplatin. When the primary site is in the skin, it is referred to as a Merkel-cell carcinoma.[31]
Extrapulmonary small-cell carcinoma localized in the lymph nodes[]
This is an extremely rare type of small cell, and there has been little information in the scientific community. It appears to occur in only one or more lymph nodes, and nowhere else in the body. Treatment is similar to small cell lung cancer, but survival rates are much higher than other small-cell carcinomas.[32]
Small-cell carcinoma of the prostate[]
Small-cell carcinoma of the prostate (SCCP) is a rare form of prostate cancer (approx. 1% of PC).[33] Due to the fact that there is little variation in prostate specific antigen levels, SCCP is normally diagnosed at an advanced stage, after metastasis.
Symptomatic metastasis of SCCP to the brain is rare, and carries a poor prognosis.[34]
Genetics[]
TP53 is mutated in 70 to 90% of SCLCs. RB1 and the retinoblastoma pathway are inactivated in most SCLCs. PTEN is mutated in 2 to 10%. MYC amplifications and amplification of MYC family members are found in 30% of SCLCs. Loss of heterozygocity on chromosome arm 3p is found in more than 80% of SCLCs, including the loss of FHIT.[35] One hundred translocations have so far been reported in SCLCs (see the "Mitelman Database"[36] and the Atlas of Genetics and Cytogenetics in Oncology and Haematology,[37]).
Diagnosis[]
When
- Localized: the cancer is confined to the lung (aka: limited stage SCLC).
- Regional: the cancer has spread to lymph nodes (or glands) within the chest (between limited and extensive stage SCLC). Lymph nodes act as a filtering system outside the lung, collecting cancer cells that are beginning to migrate out of the lung.
- Distant: the cancer has spread (or metastasized) to other parts of the body (aka: extensive stage SCLC).[38]
At the time of diagnosis, 60–70% of people already have metastases.[24]
Small-cell carcinoma is an undifferentiated neoplasm composed of primitive-appearing cells. As the name implies, the cells in small-cell carcinomas are smaller than normal cells, and barely have room for any cytoplasm. Some researchers identify this as a failure in the mechanism that controls the size of the cells.[39]
Treatment[]
Small-cell lung cancer is most commonly treated with a combination of two drugs, which is more effective than one drug alone.
Chemotherapy[]
- Cisplatin and etoposide,
- Carboplatin and etoposide.
Cisplatin-resistance[]
The drug paclitaxel may be useful in the treatment of cisplatin-resistant cancer. About 68.1% of cisplatin-resistant cells appear to be sensitive to paclitaxel and 66.7% of paclitaxel-resistant cells to cisplatin. The mechanism for this activity is unknown.[40] Paclitaxel-based chemotherapy showed modest activity in SCLC patients refractory to both etoposide- and camptothecin-based chemotherapy.[41] The newer agent lurbinectedin is active in relapsed SCLC and was approved for medical use in the United States in June 2020.[42][43][44][45][46]
Immunotherapy[]
In 2018, the FDA approved two immunotherapies for small cell lung cancer:
1. Nivolumab (Opdivo),[47][48] and
2. Atezolizumab (Tecentriq) [49][50]
Funding controversy[]
Tecentriq treatment costs on average $13,200 per month, depending on the dosage schedule.[51] Despite updated data showing 30% more people with extensive stage small cell lung cancer are alive at 24 months compared to those who received chemotherapy alone,[52] Canadian regulator had rejected to fund Tecentriq for extensive stage small-cell lung cancer "as too costly" followed by United Kingdom also citing "drug’s cost-effectiveness."[53][54]
Radiation therapy[]
Chest radiation helps SCLC patients live longer by killing cancer cells and helping prevention of cancer recurrence.[55] Another type of radiation, prophylactic cranial radiation, prevents central nervous system recurrence and can improve survival in patients with good performance status who have had a complete response or a very good partial response to chemoradiation in LD or chemotherapy in ED.[56]
In case of relapse[]
If small cell lung cancer comes back after treatment, the following combination of drugs may be used as a salvage therapy:[57]
- Cyclophosphamide (Cytoxan, Procytox),
- Doxorubicin (Adriamycin) and
- Vincristine (Oncovin)
- Paclitaxel (Taxol)
- Irinotecan (Camptosar) [58]
Current guidelines recommend that patients who relapse > 6 months from initial therapy should be retreated with the original chemotherapy regimen. For patients who relapse in < 6 months, single-agent chemotherapy either topotecan second-line therapy, or paclitaxel can be used.[59]
Novel agents[]
Several newer agents, including temozolomide and bendamustine, have activity in relapsed SCLC. Of note, temozolomide yielded a response rate of 38% for brain metastases due to SCLC.[59]
In a clinical trial of 50 patients, combination of olaparib and temozolomide in relapsed small-cell lung cancer yielded an overall response rate of 41.7%, median progression-free survival 4.2 months, and overall survival was 8.5 months.[60]
Lurbinectedin is the most promising new agent that substantially increased overall survival rate in relapsed small cell lung cancer among sensitive disease patients. As a single agent, lurbinectedin demonstrated following clinical results in refractory small cell lung cancer trial:
- Overall survival rate of 15.2 months for sensitive disease (chemotherapy-free interval of ≥ 90 days) with a disease control rate of 79.3% and overall response rate of 46.6%, and
- Overall survival rate of 5.1 months for resistant (chemotherapy-free interval of < 90 days) with a disease control rate of 46.8% and overall response rate 21.3%.[61]
Lurbinectedin is also being investigated in combination with doxorubicin as second-line therapy in a randomized phase 3 trial. While overall survival in this trial is not yet known, response rates at second line were
- 91.7% in sensitive disease with median progression-free survival of 5.8 months , and
- 33.3% in resistant disease with median progression-free of 3.5 months.[62]
Lurbinectedin is currently available in the U.S. under an expanded access program (EAP).[63][62][64]
Trilaciclib, a CKD4/6 inhibitor, reduces chemotheraphy-induced toxicity in patients being treated for small-cell lung cancer.[65][66][67] Trilaciclib’s developer, G1 Therapeutics, makes the drug available in the U.S. under expanded access while the FDA considers its New Drug Application (NDA). An approval decision on the NDA is expected by February 15, 2021.[68] On February 12, 2021, the FDA approved trilaciclib (brand name Cosela) as a treatment to reduce the frequency of chemotherapy-induced myelosuppression for patients receiving certain types of chemotherapy for extensive-stage small-cell lung cancer.[69]
Prognosis[]
5-year survival rates for small cell lung cancer (extensive and limited) range between 3.6% and 32.2% for women, and between 2.2% and 24.5% for men.[70] Relative 5-year survival rate for both sexes has increased from 3.6% in 1975 to 6.7% in 2014.[71]
Small-cell carcinoma is very responsive to chemotherapy and radiotherapy, and in particular, regimens based on platinum-containing agents. However, most people with the disease relapse and median survival remains low. The overall incidence and mortality rates of SCLC in the United States have decreased during the past few decades.[72]

In limited-stage disease, relative 5-year survival rate (both sexes, all races, all ages) is 21.3%; however, women have higher 5-year survival rates, 26.9%, and men have lower survival rates, 21.3%.[74]
The prognosis is far grimmer in extensive-stage small-cell lung carcinoma where 5-year relative survival rate (both sexes, all races, all ages) is 2.8%; however, women have higher 5-year survival rates, 3.4%, and men have lower 5-year survival rates, 2.2%.[74]
Long term survival of more than 5 years can be achieved with proper treatment. According to the 17th World Conference on Lung Cancer (WCLC), "patients who received chest radiation and prophylactic cranial irradiation along with a mean of five chemotherapy cycles could achieve a median survival of more than 5 years."[75][70]
In some cases, long term survival of 10+ years is achieved with chemotherapy and radiation alone.[76][77]
5-year survival rates[]
The SEER database tracks 5-year relative survival rates based on age, sex, and race and is considered the most accurate source of survival information.[78] This database uses terms "Localized," "Regional," and "Distant" to describe various stages of small cell lung cancer.
5-year relative survival rate for "both sexes" and "all races" affected by
- "Localized" small cell lung cancer is 28.5%;
- "Regional" small cell lung cancer 14.9%; and for
- "Distant" small cell lung cancer 2.9%.[70]
Survival rates by sex[]
Women affected by small-cell lung cancer have higher 5-year survival rates than men.[79]
- Localized: Women – 32.2% | Men – 24.5%
- Regional: Women – 17.0% | Men – 12.3%
- Distant: Women – 3.6% | Men – 2.2%
Survival rates by race, sex, age[]
5-year relative survival statistics are more accurate and, in some cases, higher when specific race and age range are combined with sex and stage at diagnosis. For example,
- Black / Female | Ages < 50 (at diagnosis) | Distant (ES SCLC) | = 7.0%.[80]
- White / Female | Ages < 50 (at diagnosis) | Distant (ES SCLC) | = 6.1% [81]
National Cancer Institute's SEER maintains publicly accessible database for specific survival rates.[82]
Epidemiology[]
15% of lung cancers in the US are of this type.[83] Small cell lung cancer occurs almost exclusively in smokers – most commonly in heavy smokers and rarely in non-smokers.[84][85]
Recalcitrant Cancer Research Act[]
In 2013, the US Congress passed the , which mandated increased attention to certain recalcitrant cancers, including small cell lung cancer. That led to the National Cancer Institute supporting small cell–specific research through a consortium.
As a result, new experimental drugs for small cell lung cancer are currently being tested, including () and Keytruda (pembrolizumab).[86][87][88][89]
Additional images[]

Anaplastic (microcellular, oat cell) carcinoma from the lung (histopathology)

Histopathologic image of small-cell carcinoma of the lung. CT-guided core needle biopsy.
Notable cases[]
- Dustin Diamond, perhaps best known as an actor on Saved by the Bell.[90]
See also[]
References[]
- ^ "small-cell carcinoma" at Dorland's Medical Dictionary
- ^ Nasu K, Hirakawa T, Okamoto M, et al. (2011). "Advanced small cell carcinoma of the uterine cervix treated by neoadjuvant chemotherapy with irinotecan and cisplatin followed by radical surgery". Rare Tumors. 3 (1): 18–20. doi:10.4081/rt.2011.e6. PMC 3070456. PMID 21464879.
- ^ Capizzello A, Peponi E, Simou N, et al. (2011). "Pure small cellliterature review". Case Rep Oncol. 4 (1): 88–95. doi:10.1159/000324717. PMC 3072185. PMID 21475596.
- ^ National Organization for Rare Disorders – Small Cell Lung Cancer | https://rarediseases.org/rare-diseases/small-cell-lung-cancer/
- ^ SEER Cancer Stats (2016) | https://seer.cancer.gov/explorer/application.php?site=611&data_type=4&graph_type=6&compareBy=stage&chk_sex_1=1&chk_race_1=1&chk_age_range_1=1&chk_stage_101=101&advopt_precision=1&showDataFor=sex_1_and_race_1_and_age_range_1
- ^ National Cancer Institute, SEER Explorer | https://seer.cancer.gov/explorer/application.php?site=611&data_type=4&graph_type=5&compareBy=sex&series=race&chk_sex_3=3&chk_race_2=2&chk_age_range_141=141&chk_stage_106=106&advopt_precision=1&showDataFor=age_range_141_and_stage_106
- ^ Chan, Bryan A.; Coward, Jermaine I. G. (2013). "Chemotherapy advances in small-cell lung cancer". Journal of Thoracic Disease. 5 (Suppl 5): S565–S578. doi:10.3978/j.issn.2072-1439.2013.07.43. ISSN 2072-1439. PMC 3804877. PMID 24163749.
- ^ Argiris A, Murren JR (2001). "Staging and clinical prognostic factors for small-cell lung cancer". Cancer J. 7 (5): 437–47. PMID 11693903.
- ^ "Limited-stage small cell lung cancer: Initial management". www.uptodate.com. UpToDate. Retrieved 2019-06-02.
- ^ "Small Cell Lung Cancer Treatment". National Cancer Institute. U.S.: National Cancer Institute, United States Department of Health and Human Services. 1980-01-01. Retrieved 2019-06-02.
- ^ Sherman CA, Rocha Lima CM, Turrisi AT (October 2000). "Limited small-cell lung cancer: a potentially curable disease". Oncology. 14 (10): 1395–403, discussion 1403–4, 1409. PMID 11098505.
- ^ Singer, Lisa; Yom, Sue S. (2015). "Consolidative radiation therapy for extensive-stage small cell lung cancer". Translational Lung Cancer Research. 4 (3): 211–14. doi:10.3978/j.issn.2218-6751.2015.04.02. ISSN 2218-6751. PMC 4483471. PMID 26207205.
- ^ Mountain CF (September 1978). "Clinical biology of small cell carcinoma: relationship to surgical therapy". Semin. Oncol. 5 (3): 272–79. PMID 211638.
- ^ Shepherd FA (February 2010). "Surgery for limited stage small cell lung cancer: time to fish or cut bait". J Thorac Oncol. 5 (2): 147–49. doi:10.1097/JTO.0b013e3181c8cbf5. PMID 20101141.
- ^ Jump up to: a b Pelayo Alvarez, Marta; Westeel, Virginie; Cortés-Jofré, Marcela; Bonfill Cosp, Xavier (27 November 2013). "Chemotherapy versus best supportive care for extensive small cell lung cancer". Cochrane Database of Systematic Reviews (11): CD001990. doi:10.1002/14651858.CD001990.pub3. PMID 24282143.
- ^ Immuno-Oncology News, "FDA Approves Opdivo for Certain Patients with Advanced Small Cell Lung Cancer" | https://immuno-oncologynews.com/2018/08/21/immunotherapy-opdivo-fda-approved-some-small-cell-lung-cancer-patients/
- ^ Horn, Leora; Mansfield, Aaron S.; Szczęsna, Aleksandra; Havel, Libor; Krzakowski, Maciej; Hochmair, Maximilian J.; Huemer, Florian; Losonczy, György; Johnson, Melissa L.; Nishio, Makoto; Reck, Martin; Mok, Tony; Lam, Sivuonthanh; Shames, David S.; Liu, Juan; Ding, Beiying; Lopez-Chavez, Ariel; Kabbinavar, Fairooz; Lin, Wei; Sandler, Alan; Liu, Stephen V. (6 December 2018). "First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer". New England Journal of Medicine. 379 (23): 2220–29. doi:10.1056/NEJMoa1809064. PMID 30280641.
- ^ Babar SM (October 2013). "SIADH associated with ciprofloxacin". The Annals of Pharmacotherapy. 47 (10): 1359–63. doi:10.1177/1060028013502457. PMID 24259701. S2CID 36759747.
- ^ Jump up to: a b Titulaer MJ, Verschuuren JJ (2008). "Lambert-Eaton myasthenic syndrome: tumor versus nontumor forms". Ann. N. Y. Acad. Sci. 1132 (1): 129–34. Bibcode:2008NYASA1132..129T. doi:10.1196/annals.1405.030. PMID 18567862. S2CID 22482871.
- ^ The Mesothelioma Center | https://www.asbestos.com/mesothelioma/small-cell/
- ^ Champaneria MC, Modlin IM, Kidd M, Eick GN (2006). "Friedrich Feyrter: a precise intellect in a diffuse system". Neuroendocrinology. 83 (5–6): 394–404. doi:10.1159/000096050. PMID 17028417. S2CID 25627846.
- ^ Jump up to: a b Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson (2007). "Ch. 13, box on morphology of small-cell lung carcinoma". Robbins Basic Pathology (8th ed.). Philadelphia: Saunders. ISBN 978-1-4160-2973-1.
- ^ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease. St. Louis, Mo: Elsevier Saunders. p. 759. ISBN 978-0-7216-0187-8.
- ^ Jump up to: a b Tammela, Tuomas; Sage, Julien (2020). "Investigating Tumor Heterogeneity in Mouse Models". Annual Review of Cancer Biology. 4: 99–119. doi:10.1146/annurev-cancerbio-030419-033413.
- ^ Jump up to: a b c Travis, William D; Brambilla, Elisabeth; Muller-Hermelink, H Konrad; et al., eds. (2004). Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart (PDF). World Health Organization Classification of Tumours. Lyon: IARC Press. ISBN 978-92-832-2418-1. Archived (PDF) from the original on 23 August 2009. Retrieved 27 March 2010.
- ^ Pelosi G, Sonzogni A, Galetta D, et al. (April 2011). "Combined small-cell carcinoma of the lung with quadripartite differentiation of epithelial, neuroendocrine, skeletal muscle, and myofibroblastic type". Virchows Arch. 458 (4): 497–503. doi:10.1007/s00428-010-1011-8. PMID 21210145. S2CID 19159554.
- ^ Gotoh M, Yamamoto Y, Huang CL, Yokomise H (November 2004). "A combined small cell carcinoma of the lung containing three components: small cell, spindle cell and squamous cell carcinoma". Eur J Cardiothorac Surg. 26 (5): 1047–49. doi:10.1016/j.ejcts.2004.08.002. PMID 15519208.
- ^ "Lambert-Eaton Myasthenic Syndrome". The Lecturio Medical Concept Library. Retrieved 1 August 2021.
- ^ Ismaili N (November 2011). "A rare bladder cancer – small cell carcinoma: review and update". Orphanet Journal of Rare Diseases. 6 (75): 75. doi:10.1186/1750-1172-6-75. PMC 3253713. PMID 22078012.
- ^ Extrapulmonary Small Cell Carcinoma at eMedicine
- ^ "Merkel-cell carcinima". Dynamed. Retrieved 2021-08-01.
- ^ Cicin, Irfan; Usta, Ufuk; Karagol, Hakan; Uzunoglu, Sernaz; Kocak, Zafer (2009). "Extrapulmonary small cell carcinoma localized in lymph nodes: Is it a different clinical entity?". Acta Oncologica. 48 (3): 354–60. doi:10.1080/02841860802495370. PMID 18979286.
- ^ Nutting C, Horwich A, Fisher C, Parsons C, Dearnaley DP (June 1997). "Small-cell carcinoma of the prostate". Journal of the Royal Society of Medicine. 90 (6): 340–41. doi:10.1177/014107689709000615. PMC 1296316. PMID 9227387.
- ^ Erasmus CE, Verhagen WI, Wauters CA, van Lindert EJ (November 2002). "Brain metastasis from prostate small-cell carcinoma: not to be neglected". Can J Neurol Sci. 29 (4): 375–77. doi:10.1017/S0317167100002250. PMID 12463494.
- ^ "Lung: Translocations in Small Cell Carcinoma". atlasgeneticsoncology.org. Archived from the original on 30 January 2015. Retrieved 30 April 2018.
- ^ "Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer". Archived from the original on 2016-05-29.
- ^ "Atlas of Genetics and Cytogenetics in Oncology and Haematology". atlasgeneticsoncology.org. Archived from the original on 2011-02-23.
- ^ Lung Cancer – Cleveland Clinic | https://my.clevelandclinic.org/health/diseases/4375-lung-cancer
- ^ Leslie M (November 2011). "Mysteries of the cell. How does a cell know its size?". Science. 334 (6059): 1047–48. doi:10.1126/science.334.6059.1047. PMID 22116854.
- ^ Stordal, B.; Pavlakis, N.; Davey, R. (December 2007). "A systematic review of platinum and taxane resistance from bench to clinic: an inverse relationship" (PDF). Cancer Treat. Rev. 33 (8): 688–703. doi:10.1016/j.ctrv.2007.07.013. hdl:2123/4068. PMID 17881133.
- ^ Kim, S. H., Kim, M. J., Kim, Y. J., Chang, H., Kim, J. W., Lee, J. O., Lee, K. W., Kim, J. H., Bang, S. M., & Lee, J. S. (2017). Paclitaxel as third-line chemotherapy for small cell lung cancer failing both etoposide- and camptothecin-based chemotherapy. Medicine, 96(42), e8176. https://doi.org/10.1097/MD.0000000000008176
- ^ "Zepzelca: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 15 June 2020.
- ^ "Zepzelca- lurbinectedin injection, powder, lyophilized, for solution". DailyMed. 15 June 2020. Retrieved 24 September 2020.
- ^ "Jazz Pharmaceuticals Announces U.S. FDA Accelerated Approval of Zepzelca (lurbinectedin) for the Treatment of Metastatic Small Cell Lung Cancer" (Press release). Jazz Pharmaceuticals. 15 June 2020. Retrieved 15 June 2020 – via PR Newswire.
- ^ "FDA grants accelerated approval to lurbinectedin for metastatic small". U.S. Food and Drug Administration (FDA). 15 June 2020. Retrieved 16 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Drug Trials Snapshots: Zepzelca". U.S. Food and Drug Administration (FDA). 15 June 2020. Retrieved 28 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "FDA Approves Opdivo for Small Cell Lung Cancer Treatment". Cure Today.
- ^ FDA Approves Opdivo (Nivolumab) for Small Cell Lung Cancer | https://www.cancer.org/latest-news/fda-approves-opdivo-nivolumab-for-small-cell-lung-cancer.html
- ^ FDA approves atezolizumab for extensive-stage small cell lung cancer | https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm633814.htm
- ^ Concurrent Tecentriq Adds First Survival Benefit Seen in Small Cell Lung Cancer in 20 Years | https://www.curetoday.com/articles/concurrent-tecentriq-adds-first-survival-benefit-seen-in-small-cell-lung-cancer-in-20-years
- ^ Drugs.com – What is the cost of Tecentriq?| https://www.drugs.com/medical-answers/cost-tecentriq-3064818/
- ^ Tecentriq: "Updated exploratory OS analysis" | https://www.tecentriq-hcp.com/sclc/clinical-data-efficacy/study-efficacy.html
- ^ Reuters, "Canadian regulator considers changes to new drug pricing plan", Feb 20, 2020. https://ca.reuters.com/article/domesticNews/idCAKBN20E2LI
- ^ Reuters, "NICE Cites Cost in Deciding Against Atezolizumab for Frontline Advanced Small Cell Lung Cancer", Jan 6, 2020 https://www.onclive.com/web-exclusives/nice-cites-cost-in-deciding-against-atezolizumab-for-frontline-advanced-small-cell-lung-cancer
- ^
- ^
- ^ [[Treatment for Small Cell Lung Cancer, Canadian Cancer Society| http://www.cancer.ca/en/cancer-information/cancer-type/lung/treatment/treatment-for-small-cell-lung-cancer/?region=nu
- ^ Mouri, Atsuto; Yamaguchi, Ou; Miyauchi, Sachiko; Shiono, Ayako; Utsugi, Harue; Nishihara, Fuyumi; Murayama, Yoshitake; Kagamu, Hiroshi; Kobayashi, Kunihiko (January 2019). "Combination therapy with carboplatin and paclitaxel for small cell lung cancer". Respiratory Investigation. 57 (1): 34–39. doi:10.1016/j.resinv.2018.09.004. PMID 30528688.
- ^ Jump up to: a b Qin, Angel; Kalemkerian, Gregory P. (June 2018). "Treatment Options for Relapsed Small-Cell Lung Cancer: What Progress Have We Made?". Journal of Oncology Practice. 14 (6): 369–70. doi:10.1200/JOP.18.00278. PMID 29894661.
- ^ Combination Olaparib and Temozolomide in Relapsed Small-Cell Lung Cancer, Cancer Discovery, October 2019. - American Association for Cancer Research https://cancerdiscovery.aacrjournals.org/content/9/10/1372
- ^ Efficacy and safety profile of lurbinectedin in second-line SCLC patients: Results from a phase II single-agent trial. | https://meetinglibrary.asco.org/record/173489/abstract
- ^ Jump up to: a b Calvo, E.; Moreno, V.; Flynn, M.; Holgado, E.; Olmedo, M.E.; Lopez Criado, M.P.; Kahatt, C.; Lopez-Vilariño, J.A.; Siguero, M.; Fernandez-Teruel, C.; Cullell-Young, M.; Soto Matos-Pita, A.; Forster, M. (October 2017). "Antitumor activity of lurbinectedin (PM01183) and doxorubicin in relapsed small-cell lung cancer: results from a phase I study". Annals of Oncology. 28 (10): 2559–66. doi:10.1093/annonc/mdx357. PMC 5834091. PMID 28961837.
- ^ Emas, Bionical. "PharmaMar and Bionical Emas Launch Expanded Access Program for Lurbinectedin in Relapsed Small Cell Lung Cancer in the U.S." www.prnewswire.com.
- ^ Farago, Anna F; Drapkin, Benjamin J; Lopez-Vilarino de Ramos, Jose Antonio; Galmarini, Carlos M; Núñez, Rafael; Kahatt, Carmen; Paz-Ares, Luis (January 2019). "ATLANTIS: a Phase III study of lurbinectedin/doxorubicin versus topotecan or cyclophosphamide/doxorubicin/vincristine in patients with small-cell lung cancer who have failed one prior platinum-containing line". Future Oncology. 15 (3): 231–39. doi:10.2217/fon-2018-0597. PMC 6331752. PMID 30362375.
- ^ Weiss, J. M.; Csoszi, T.; Maglakelidze, M.; Hoyer, R. J.; Beck, J. T.; Gomez, M. Domine; Lowczak, A.; Aljumaily, R.; Lima, C. M. Rocha; Boccia, R. V.; Hanna, W. (2019-10-01). "Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized phase II trial". Annals of Oncology. 30 (10): 1613–21. doi:10.1093/annonc/mdz278. ISSN 0923-7534. PMC 6857609. PMID 31504118.
- ^ "FDA Grants Priority Review to Trilaciclib to Treat Patients with SCLC". Cancer Network. Retrieved 2020-12-04.
- ^ "Trilaciclib | intravenous CDK4/6 inhibitor | G1 Therapeutics, Inc". www.g1therapeutics.com. Retrieved 2020-12-28.
- ^ staff, By. "FDA Grants Priority Review of Trilaciclib for Treating Small Cell Lung Cancer". www.uspharmacist.com. Retrieved 2020-12-28.
- ^ Commissioner, Office of the (2021-02-12). "FDA Approves Drug to Reduce Bone Marrow Suppression Caused by Chemotherapy". FDA. Retrieved 2021-02-16.
- ^ Jump up to: a b c the SEER Cancer Statistics Review 1975-2015 | https://seer.cancer.gov/csr/1975_2015/browse_csr.php?sectionSEL=15&pageSEL=sect_15_table.13
- ^ "Table 15.13 Small Cell Cancer of the Lung and Bronchus (Invasive) 5-Year Relative and Period Survival by Race, Sex, Diagnosis Year, Age and Stage at Diagnosis". National Cancer Institute. Retrieved 26 June 2021.
- ^ [https://www.oncolink.org/healthcare-professionals/nci/pqid-CDR00000629452%7C National Cancer Institute: Small Cell Lung Cancer Treatment
- ^ Smokers defined as current or former smoker of more than 1 year of duration. See image page in Commons for percentages in numbers. Reference:
- Table 2 Archived 2017-09-10 at the Wayback Machine in: Kenfield SA, Wei EK, Stampfer MJ, Rosner BA, Colditz GA (2008). "Comparison of aspects of smoking among the four histological types of lung cancer". Tob Control. 17 (3): 198–204. doi:10.1136/tc.2007.022582. PMC 3044470. PMID 18390646.
- ^ Jump up to: a b [2016 Cancer Stats by SEER | https://seer.cancer.gov/explorer/application.php?site=611&data_type=4&graph_type=6&compareBy=stage&chk_sex_2=2&chk_race_1=1&chk_age_range_1=1&chk_stage_104=104&advopt_precision=1&showDataFor=sex_2_and_race_1_and_age_range_1
- ^ Medscape "Exceptional SCLC Survivors: 5-Year Median Survival", Dec 20, 2016. | https://www.medscape.com/viewarticle/873492
- ^
- ^ Extensive Stage SCLC survivor Montessa M. Lee was presenting during 2019 IASLC | https://www.iaslc.org/news/focus-immunotherapy-and-patient-perspective-highlight-iaslc-2019-small-cell-lung-cancer-meetin]]
- ^ 5-Year Relative Survival (Percent) 2008–2014 by Stage at Diagnosis | https://seer.cancer.gov/csr/1975_2015/browse_csr.php?sectionSEL=15&pageSEL=sect_15_table.13#table5
- ^ "Browse the Tables and Figures – SEER Cancer Statistics Review (CSR) 1975–2015".
- ^ [SEER Database, Cancer Survival Rates | https://seer.cancer.gov/explorer/application.php?site=611&data_type=4&graph_type=5&compareBy=age_range&series=sex&chk_sex_3=3&chk_race_3=3&chk_age_range_9=9&chk_stage_106=106&advopt_precision=1&advopt_show_se=on&advopt_show_ci=on&showDataFor=race_3_and_stage_106]
- ^ [SEER Database, Cancer Survival Rates | https://seer.cancer.gov/explorer/application.php?site=611&data_type=4&graph_type=5&compareBy=age_range&series=sex&chk_sex_3=3&chk_race_8=8&chk_age_range_9=9&chk_stage_106=106&advopt_precision=1&advopt_show_se=on&advopt_show_ci=on&showDataFor=race_8_and_stage_106]
- ^ [SEER Small Cell Lung Cancer Survival Rates | https://seer.cancer.gov/explorer/application.php?site=611&data_type=4&graph_type=6&compareBy=stage&chk_sex_3=3&chk_race_1=1&chk_age_range_1=1&chk_stage_101=101&advopt_precision=1&showDataFor=sex_3_and_race_1_and_age_range_1]
- ^ World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.1. ISBN 978-9283204299.
- ^ Ettinger, DS; Aisner, J (1 October 2006). "Changing face of small-cell lung cancer: real and artifact". Journal of Clinical Oncology. 24 (28): 4526–27. doi:10.1200/jco.2006.07.3841. PMID 17008688.
- ^ Muscat, JE; Wynder, EL (6 January 1995). "Lung cancer pathology in smokers, ex-smokers and never smokers". Cancer Letters. 88 (1): 1–5. doi:10.1016/0304-3835(94)03608-l. PMID 7850764.
- ^ Fred Hutch "A new focus on small cell lung cancer" | https://www.fredhutch.org/en/news/center-news/2019/02/q-a-with-david-macpherson-lung-cancer.html
- ^ Augert, Arnaud; Eastwood, Emily; Ibrahim, Ali H.; Wu, Nan; Grunblatt, Eli; Basom, Ryan; Liggitt, Denny; Eaton, Keith D.; Martins, Renato; Poirier, John T.; Rudin, Charles M.; Milletti, Francesca; Cheng, Wei-Yi; Mack, Fiona; MacPherson, David (5 February 2019). "Targeting NOTCH activation in small cell lung cancer through LSD1 inhibition". Science Signaling. 12 (567): eaau2922. doi:10.1126/scisignal.aau2922. PMC 6530478. PMID 30723171.
- ^ Oryzon Genomics "ORY-1001 – Patient Info" | https://www.oryzon.com/en/patient/patient-information
- ^ Cure Magazine "Keytruda Shows Benefit in Small Cell Lung Cancer" | https://www.curetoday.com/articles/keytruda-shows-benefit-in-small-cell-lung-cancer
- ^ "Dustin Diamond, 'Saved by the Bell' Star, Dead at 44". Entertainment Tonight. Retrieved 2021-02-06.
| Classification | |
|---|---|
| External resources |
- Carcinoma
- Cytopathology
- Infectious causes of cancer
- Lung cancer
- Rare diseases

