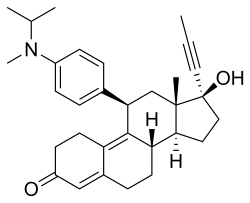
Toripristone
 | |
| Clinical data | |
|---|---|
| Other names | RU-40555; 17β-Hydroxy-11β-[4-[methyl(1-methylethyl)amino]phenyl]-17α-(1-propyn-1-yl)estra-4,9-dien-3-one |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C31H39NO2 |
| Molar mass | 457.658 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Toripristone (INN) (developmental code name RU-40555) is a synthetic, steroidal antiglucocorticoid as well as antiprogestogen which was never marketed.[1][2] It is reported as a potent and highly selective antagonist of the glucocorticoid receptor (GR) (Ki = 2.4 nM),[3][4] though it also acts as an antagonist of the progesterone receptor (PR).[5][6] The pharmacological profile of toripristone is said to be very similar to that of mifepristone, except that toripristone does not bind to orosomucoid (α1-acid glycoprotein).[5] The drug has been used to study the hypothalamic-pituitary-adrenal axis and has been used as a radiotracer for the GR.[4][3] Its INN was given in 1990.[2]
See also[]
- Aglepristone
- Lilopristone
- Onapristone
- Telapristone
References[]
- ^ R.A. Hill; H.L.J. Makin; D.N. Kirk; G.M. Murphy (23 May 1991). Dictionary of Steroids. CRC Press. pp. 101–. ISBN 978-0-412-27060-4.
- ^ a b https://mednet-communities.net/inn/db/media/docs/p-innlist61.pdf
- ^ a b Steiniger, Bjorn; Kniess, Torsten; Bergmann, Ralf; Pietzsch, Jens; Wuest, Frank (2008). "Radiolabeled Glucocorticoids as Molecular Probes for Imaging Brain Glucocorticoid Receptors by Means of Positron Emission Tomography (PET)". Mini-Reviews in Medicinal Chemistry. 8 (7): 728–739. doi:10.2174/138955708784567403. ISSN 1389-5575. PMID 18537728.
- ^ a b Bachmann CG, Linthorst AC, Holsboer F, Reul JM (2003). "Effect of chronic administration of selective glucocorticoid receptor antagonists on the rat hypothalamic-pituitary-adrenocortical axis". Neuropsychopharmacology. 28 (6): 1056–67. doi:10.1038/sj.npp.1300158. PMID 12700716.
- ^ a b Philibert, D.; Costerousse, G.; Gaillard-Moguilewsky, M.; Nedelec, L.; Nique, F.; Tournemine, C.; Teutsch, G. (1991). "From RU 38486 towards Dissociated Antiglucocorticoid and Antiprogesterone". Antihormones in Health and Disease. Frontiers of Hormone Research. Vol. 19. pp. 1–17. doi:10.1159/000419634. ISBN 978-3-8055-5297-4. ISSN 0301-3073.
- ^ Fu, Jing; Hu, Lina; Huang, Wei; Zhu, Huili; Wang, Qiushi; He, Fan; Xie, Lingxia; Gan, Xiaoling; Huang, Wei (2012). "Progesterone receptor antagonists and progesterone receptor modulators for endometriosis". In Huang, Wei (ed.). Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD009881.
Categories:
- 1-Ethynylcyclopentanols
- Amines
- Antiglucocorticoids
- Antiprogestogens
- Estranes
- Enones
- Steroid stubs
- Genito-urinary system drug stubs