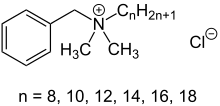
Benzalkonium chloride
| |
| Names | |
|---|---|
| Other names
N-Alkyl-N-benzyl-N,N-dimethylammonium chloride; Alkyldimethylbenzylammonium chloride; ADBAC; BC50 BC80; Quaternary ammonium compounds; quats
| |
| Identifiers | |
| |
| ChEBI | |
| ChEMBL | |
| EC Number |
|
| KEGG | |
| RTECS number |
|
| UNII | |
| Properties | |
| variable | |
| Molar mass | variable |
| Appearance | 100% is white or yellow powder; gelatinous lumps; Solutions BC50 (50%) & BC80 (80%) are colourless to pale yellow solutions |
| Density | 0.98 g/cm3 |
| very soluble | |
| Pharmacology | |
| D08AJ01 (WHO) | |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
GHS hazard statements
|
H302, H312, H314, H318, H400, H410 |
| P260, P264, P270, P273, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P322, P330, P363, P391, P405, P501 | |
| NFPA 704 (fire diamond) | 
3
0
0 |
| Flash point | 250 °C (482 °F; 523 K) (if solvent based) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benzalkonium chloride (BZK, BKC, BAK, BAC), also known as alkyldimethylbenzylammonium chloride (ADBAC) and by the trade name Zephiran,[1] is a type of cationic surfactant. It is an organic salt classified as a quaternary ammonium compound. ADBACs have three main categories of use: as a biocide, a cationic surfactant, and a phase transfer agent.[2] ADBACs are a mixture of alkylbenzyldimethylammonium chlorides, in which the alkyl group has various even-numbered alkyl chain lengths.
Solubility and physical properties[]
Depending on purity, benzalkonium chloride ranges from colourless to a pale yellow (impure). Benzalkonium chloride is readily soluble in ethanol and acetone. Dissolution in water is ready, upon agitation. Aqueous solutions should be neutral to slightly alkaline. Solutions foam when shaken. Concentrated solutions have a bitter taste and a faint almond-like odour.[citation needed]
Standard concentrates are manufactured as 50% and 80% w/w solutions, and sold under trade names such as BC50, BC80, BAC50, BAC80, etc. The 50% solution is purely aqueous, while more concentrated solutions require incorporation of rheology modifiers (alcohols, polyethylene glycols, etc.) to prevent increases in viscosity or gel formation under low temperature conditions.
Cationic surfactant[]
Benzalkonium chloride also possesses surfactant properties, dissolving the lipid phase of the tear film and increasing drug penetration, making it a useful excipient, but at the risk of causing damage to the surface of the eye.[3]
- Laundry detergents and treatments.
- Softeners for textiles.
Phase transfer agent[]
Benzalkonium chloride is a mainstay of phase-transfer catalysis, an important technology in the synthesis of organic compounds, including drugs.
Bioactive agents[]
Especially for its antimicrobial activity, benzalkonium chloride is an active ingredient in many consumer products:
- Pharmaceutical products such as eye, ear and nasal drops or sprays, as a preservative.
- Personal care products such as hand sanitizers, wet wipes, shampoos, soaps, deodorants and cosmetics.[4]
- Skin antiseptics and wound wash sprays, such as Bactine.[5][6]
- Throat lozenges[7] and mouthwashes, as a biocide
- Spermicidal creams.
- Cleaners for floor and hard surfaces as a disinfectant, such as Lysol and Dettol antibacterial spray and wipes.
- Algaecides for clearing of algae, moss, lichens from paths, roof tiles, swimming pools, masonry, etc.
Benzalkonium chloride is also used in many non-consumer processes and products, including as an active ingredient in surgical disinfection. A comprehensive list of uses includes industrial applications.[8] An advantage of benzalkonium chloride, not shared by ethanol-based antiseptics or hydrogen peroxide antiseptic, is that it does not cause a burning sensation when applied to broken skin.[citation needed] However, prolonged or repeated skin contact may cause dermatitis.[9]
During the course of the COVID-19 pandemic, from time to time there have been shortages of hand cleaner containing ethanol or isopropanol as active ingredients. The FDA has stated that benzalkonium chloride is eligible as an alternative for use in the formulation of healthcare personnel hand rubs.[10] However, in reference to the FDA rule, the CDC states that it does not have a recommended alternative to ethanol or isopropanol as active ingredients, and adds that "available evidence indicates benzalkonium chloride has less reliable activity against certain bacteria and viruses than either of the alcohols."[11] In November 2020 The Journal of Hospital Infection published a study on benzalkonium chloride formulations; it was found that laboratory and commercial disinfectants with 0.13% to 0.28% inactivated the SARS-CoV-2 virus within 15 seconds of contact, even in the presence of a soil or hard water.[12] This resulted in a growing consensus that BZK sanitizers are just as effective as alcohol-based sanitizers despite the CDC guidelines.[13] As a hand sanitizer, use of BZK may be advantageous over ethanol in some situations because it has significantly more residual antibacterial action on the skin after initial application.[14] Benzalkonium chloride has demonstrated persistent antimicrobial activity for up to four hours after contact whereas ethanol-based sanitizer demonstrate skin protection for only 10 minutes post-application.[15]
Medicine[]
Benzalkonium chloride is a frequently used preservative in eye drops; typical concentrations range from 0.004% to 0.01%. Stronger concentrations can be caustic[16] and cause irreversible damage to the corneal endothelium.[17]
Avoiding the use of benzalkonium chloride solutions while contact lenses are in place is discussed in the literature.[18][19]
Due to its antimicrobial activity[20] when applied to skin, some topical medications for acne vulgaris have benzalkonium chloride added to increase the products’ efficiency or shelf-life.[21][22]
In Russia and China, benzalkonium chloride is used as a contraceptive. Tablets are inserted vaginally, or a gel is applied, resulting in local spermicidal contraception.[23][24] It is not a failsafe method, and can cause irritation.
Beekeeping[]
It is used in beekeeping for the treatment of rotten diseases of the brood.[25]
Adverse effects[]
Although historically benzalkonium chloride has been ubiquitous as a preservative in ophthalmic preparations, its ocular toxicity and irritant properties,[26] in conjunction with consumer demand, have led pharmaceutical companies to increase production of preservative-free preparations, or to replace benzalkonium chloride with preservatives which are less harmful.[citation needed]
Many mass-marketed inhaler and nasal spray formulations contain benzalkonium chloride as a preservative, despite substantial evidence that it can adversely affect ciliary motion, mucociliary clearance, nasal mucosal histology, human neutrophil function, and leukocyte response to local inflammation.[27] Although some studies have found no correlation between use of benzalkonium chloride in concentrations at or below 0.1% in nasal sprays and drug-induced rhinitis,[28] others have recommended that benzalkonium chloride in nasal sprays be avoided.[29][30] In the United States, nasal steroid preparations that are free of benzalkonium chloride include budesonide, triamcinolone acetonide, dexamethasone, and Beconase and Vancenase aerosol inhalers.[27]
Benzalkonium chloride is irritant to middle ear tissues at typically used concentrations. Inner ear toxicity has been demonstrated.[31]
Occupational exposure to benzalkonium chloride has been linked to the development of asthma.[32] In 2011, a large clinical trial designed to evaluate the efficacy of hand sanitizers based on different active ingredients in preventing virus transmission amongst schoolchildren was re-designed to exclude sanitizers based on benzalkonium chloride due to safety concerns.[33]
Benzalkonium chloride has been in common use as a pharmaceutical preservative and antimicrobial since the 1940s. While early studies confirmed the corrosive and irritant properties of benzalkonium chloride, investigations into the adverse effects of, and disease states linked to, benzalkonium chloride have only surfaced during the past 30 years.[citation needed]
Toxicology[]
RTECS lists the following acute toxicity data:[34]
| Organism | Route of exposure | Dose (LD50) |
|---|---|---|
| Rat | Intravenous | 13.9 mg/kg |
| Rat | Oral | 240 mg/kg |
| Rat | Intraperitoneal | 14.5 mg/kg |
| Rat | Subcutaneous | 400 mg/kg |
| Mouse | Subcutaneous | 64 mg/kg |
Benzalkonium chloride is a human skin and severe eye irritant.[35] It is a suspected respiratory toxicant, immunotoxicant, gastrointestinal toxicant, and neurotoxicant.[36][37][38]
Benzalkonium chloride formulations for consumer use are dilute solutions. Concentrated solutions are toxic to humans, causing corrosion/irritation to the skin and mucosa, and death if taken internally in sufficient volumes. 0.1% is the maximum concentration of benzalkonium chloride that does not produce primary irritation on intact skin or act as a sensitizer.[39]
Poisoning by benzalkonium chloride is recognised in the literature.[40] A 2014 case study detailing the fatal ingestion of up to 8.1 oz (240ml) of 10% benzalkonium chloride in a 78-year-old male also includes a summary of the currently published case reports of benzalkonium chloride ingestion. While the majority of cases were caused by confusion about the contents of containers, one case cites incorrect pharmacy dilution of benzalkonium chloride as the cause of poisoning of two infants.[41] In 2018 a Japanese nurse was arrested and admitted to having poisoned approximately 20 patients at a hospital in Yokohama by injecting benzalkonium chloride into their intravenous drip bags.[42][43]
Benzalkonium chloride poisoning of domestic pets has been recognised as a result of direct contact with surfaces cleaned with disinfectants using benzalkonium chloride as an active ingredient.[44]
Biological activity[]
The greatest biocidal activity is associated with the C12 dodecyl and C14 myristyl alkyl derivatives. The mechanism of bactericidal/microbicidal action is thought to be due to disruption of intermolecular interactions. This can cause dissociation of cellular membrane lipid bilayers, which compromises cellular permeability controls and induces leakage of cellular contents. Other biomolecular complexes within the bacterial cell can also undergo dissociation. Enzymes, which finely control a wide range of respiratory and metabolic cellular activities, are particularly susceptible to deactivation. Critical intermolecular interactions and tertiary structures in such highly specific biochemical systems can be readily disrupted by cationic surfactants.[citation needed]
Benzalkonium chloride solutions are fast-acting biocidal agents with a moderately long duration of action. They are active against bacteria and some viruses, fungi, and protozoa. Bacterial spores are considered to be resistant. Solutions are bacteriostatic or bactericidal according to their concentration. Gram-positive bacteria are generally more susceptible than gram-negative bacteria. Its activity depends on the surfactant concentration and also on the bacterial concentration (inoculum) at the moment of the treatment.[45] Activity is not greatly affected by pH, but increases substantially at higher temperatures and prolonged exposure times.
In a 1998 study using the FDA protocol, a non-alcohol sanitizer with benzalkonium chloride as the active ingredient met the FDA performance standards, while Purell, a popular alcohol-based sanitizer, did not. The study, which was undertaken and reported by a leading US developer, manufacturer and marketer of topical antimicrobial pharmaceuticals based on quaternary ammonium compounds, found that their own benzalkonium chloride-based sanitizer performed better than alcohol-based hand sanitizer after repeated use.[46]
Advancements in the quality and efficacy of benzalkonium chloride in current non-alcohol hand sanitizers has addressed the CDC concerns regarding gram negative bacteria, with the leading products being equal if not more effective against gram negative, particularly New Delhi metallo-beta-lactamase 1 and other antibiotic resistant bacteria.[citation needed]
Newer formulations using benzalkonium blended with various quaternary ammonium derivatives can be used to extend the biocidal spectrum and enhance the efficacy of benzalkonium based disinfection products.[citation needed] Formulation techniques have been used to great effect in enhancing the virucidal activity of quaternary ammonium-based disinfectants such as Virucide 100 to typical healthcare infection hazards such as hepatitis and HIV.[citation needed] The use of appropriate excipients can also greatly enhance the spectrum, performance and detergency, and prevent deactivation under use conditions.[citation needed] Formulation can also help minimise deactivation of benzalkonium solutions in the presence of organic and inorganic contamination.[citation needed]
Degradation[]
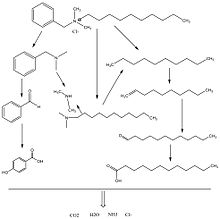
Benzalkonium chloride degradation follows consecutive debenzylation, dealkylation, and demethylation steps producing benzyl chloride, alkyl dimethyl amine, dimethyl amine, long chain alkane, and ammonia.[47] The intermediates, major, and minor products can then be broken down into CO2, H2O, NH3, and Cl–. The first step to the biodegradation of BAC is the fission or splitting of the alkyl chain from the quaternary nitrogen as shown in the diagram. This is done by abstracting the hydrogen from the alkyl chain by using a hydroxyl radical leading to a carbon centered radical. This results in benzyl dimethyl amine as the first intermediate and dodecanal as the major product.[47]
From here, benzyl dimethyl amine can be oxidized to benzoic acid using the Fenton process. The trimethyl amine group in dimethylbenzylamine can be cleaved to form a benzyl that can be further oxidized to benzoic acid. Benzoic acid uses hydroxylation (adding a hydroxyl group) to form p-hydroxybenzoic acid. Benzyldimethylamine can then be converted into ammonia by performing demethylation twice, which removes both methyl groups, followed by debenzylation, removing the benzyl group using hydrogenation.[47] The diagram represents suggested pathways of the biodegradation of BAC for both the hydrophobic and the hydrophilic regions of the surfactant. Since stearalkonium chloride is a type of BAC, the biodegradation process should happen in the same manner.
Regulation[]
Benzalkonium chloride is classed as a Category III antiseptic active ingredient by the United States Food and Drug Administration (FDA). Ingredients are categorized as Category III when "available data are insufficient to classify as safe and effective, and further testing is required”. Benzalkonium chloride was deferred from further rulemaking in the 2019 FDA Final Rule on safety and effectiveness of consumer hand sanitizers, "to allow for the ongoing study and submission of additional safety and effectiveness data necessary to make a determination" on whether it met these criteria for use in OTC hand sanitizers, but the agency indicated it did not intend to take action to remove benzalkonium chloride-based hand sanitizers from the market.[48] There is acknowledgement that more data are required on its safety, efficacy, and effectiveness, especially with relation to:
- Human pharmacokinetic studies, including information on its metabolites
- Studies on animal absorption, distribution, metabolism, and excretion
- Data to help define the effect of formulation on dermal absorption
- Carcinogenicity
- Studies on developmental and reproductive toxicology
- Potential hormonal effects
- Assessment of the potential for development of bacterial resistance
- Risks of using it as a contraceptive method
In September 2016, the FDA announced a ban on nineteen ingredients in consumer antibacterial soaps citing a lack of evidence for safety and effectiveness. A ban on three additional ingredients, including benzalkonium chloride, was deferred to allow ongoing studies to be completed.[49]
See also[]
- Stearalkonium chloride – Anti-static agent, surfactant and antimicrobial.
- Polyaminopropyl biguanide – an alternative preservative for contact lens solutions
- Ethylenediaminetetraacetic acid
- Triclosan
- Thiomersal – Organomercury antiseptic and antifungal agent
References[]
- ^ "Zephiran (benzalkonium chloride)" (PDF). Sanofi. Retrieved 28 April 2020.
- ^ Maximilian Lackner, Josef Peter Guggenbichler "Antimicrobial Surfaces" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2013. doi:10.1002/14356007.q03_q01
- ^ Bartlett, J (2013). Clinical Ocular Pharmacology (2 ed.). Elsevier. p. 20. ISBN 978-1-483-19391-5.
- ^ "Benzalkonium Chloride | Cosmetics Info". cosmeticsinfo.org. Retrieved 2021-03-10.
- ^ "Bactine® Pain Relieving Cleansing Spray". dailymed.nlm.nih.gov. Retrieved 2020-03-04.
- ^ "Bactine Original First Aid Liquid, 4 fl oz Ingredients and Reviews". SkinSAFE. Retrieved 2020-03-04.
- ^ "Bradosol". Archived from the original on 2014-10-12. Retrieved 2013-05-20.
- ^ Ash, M; Ash, I (2004). Handbook of Preservatives. Synapse Info Resources. p. 286. ISBN 978-1-890-59566-1.
- ^ "Benzalkonium chloride Material Safety Data Sheet". ScienceLab.com. 2013-05-22. Archived from the original on 2012-10-21.
- ^ "Safety and Effectiveness for Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use (Final Rule)". U.S. Food and Drug Administration. 20 December 2017.
- ^ "Hand Hygiene Recommendations; Guidance for Healthcare Providers about Hand Hygiene and COVID-19". Centers for Disease Control and Prevention. 17 May 2020.
- ^ Ogilvie, Benjamin; Solis-Leal, Antonio (November 28, 2020). "Alcohol-free hand sanitizer and other quaternary ammonium disinfectants quickly and effectively inactivate SARS-CoV-2". Journal of Hospital Infection. 108: 142–145. doi:10.1016/j.jhin.2020.11.023. ISSN 0195-6701. PMC 7700010. PMID 33259880.
- ^ Hollingshead, Todd (December 1, 2020). "Alcohol-free hand sanitizer just as effective against COVID as alcohol-based versions". EurekAlert!. BRIGHAM YOUNG UNIVERSITY: AAAS. Retrieved December 14, 2020.
- ^ Bondurant, Sidney W.; Duley, Collette M.; Harbell, John W. (August 2019). "Demonstrating the persistent antibacterial efficacy of a hand sanitizer containing benzalkonium chloride on human skin at 1, 2, and 4 hours after application". American Journal of Infection Control. 47 (8): 928–932. doi:10.1016/j.ajic.2019.01.004. ISSN 1527-3296. PMID 30777389.
- ^ Reporter, JAMES ROSEN, Sinclair Investigative (2020-04-01). "EXCLUSIVE: Sanitizer opposed by CDC kills coronavirus "surrogate" in lab tests". WJLA. Retrieved 2021-03-16.
- ^ Nelson, L; Goldfrank, L (2011). Goldfrank's Toxicologic Emergencies (9 ed.). McGraw-Hill Medical. p. 803. ISBN 978-0-071-60593-9.
- ^ Baudouin, C; Creuzot-Garcher, C; Hoang-Xuan, T (2001). Inflammatory Diseases of the Conjunctiva (1, illustrated ed.). Thieme. p. 141. ISBN 978-3-131-25871-7.
- ^ Otten, Mary; Szabocsik, John M. (1976). "Measurement of Preservative Binding with SOFLENS (polymacon) Contact Lens". Clinical and Experimental Optometry. 59 (8): 277. doi:10.1111/j.1444-0938.1976.tb01445.x.
- ^ M Akers, "Consideration in selecting antimicrobial preservative agents for parenteral product development", Pharmaceutical Technology, May, p. 36 (1984).
- ^ [1], "Antimicrobial compositions and methods for using the same", issued 1996-12-03
- ^ [2], "Composition containing anti-acne agent and method of using the same", issued 2005-07-06
- ^ "New Combination Acne Treatment Safe and Effective". Pharmacy Times. Retrieved 2021-03-10.
- ^ "ISRCTN - ISRCTN16203579: Effectiveness of BenZalKonium chloride gel as vaginal contraceptive: a multicentric randomised controlled trial". www.isrctn.com.
- ^ Li, Weihua; Huang, Zirong; Wu, Yu; Wang, Haiyun; Zhou, Xiaobo; Xia o, Zhiqin; Ding, Xuncheng; Xu, Jinxun (June 26, 2013). "Effectiveness of an optimized benzalkonium chloride gel as vaginal contraceptive: a randomized controlled trial among Chinese women". Contraception. 87 (6): 756–765. doi:10.1016/j.contraception.2012.09.012. PMID 23089047.
- ^ "БЕНЗАЛКОНИЯ ХЛОРИД (benzalkonium chloride) действующее вещество | 36i6.info". 36i6.info (in Russian). 2017-04-15. Retrieved 2019-08-28.
- ^ Baudouin, C; Labbé, A; Liang, H; Pauly, A; Brignole-Baudouin, F (2010). "Preservatives in eyedrops: the good, the bad and the ugly". Prog Retin Eye Res. 29 (4): 312–34. doi:10.1016/j.preteyeres.2010.03.001. PMID 20302969. S2CID 24575844.
- ^ Jump up to: a b Kennedy, D W; Bolger, W E; Zinreich, S J (2001). Diseases of the Sinuses Diagnosis and Management. B.C. Decker Inc. p. 162. ISBN 978-1-550-09045-1.
- ^ Marple, B; Roland, P; Benninger, M (2004). "Safety review of benzalkonium chloride used as a preservative in intranasal solutions: an overview of conflicting data and opinions". Otolaryngology–Head and Neck Surgery. 130 (1): 131–41. doi:10.1016/j.otohns.2003.07.005. PMID 14726922. S2CID 24967410.
- ^ Beule, A. G. (2010). "Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses". GMS Current Topics in Otorhinolaryngology, Head and Neck Surgery. 9: Doc07. doi:10.3205/cto000071. PMC 3199822. PMID 22073111.
- ^ Graf, P (2005). "Rhinitis medicamentosa: a review of causes and treatment". Treatments in Respiratory Medicine. 4 (1): 21–9. doi:10.2165/00151829-200504010-00003. PMID 15725047. S2CID 25175067.
- ^ Snow, J. B.; Wackym, P. A. (2009). Ballenger's Otorhinolaryngology: Head and Neck Surgery (revised ed.). PMPH-USA. p. 277. ISBN 978-1-550-09337-7.
- ^ Malo, J; Chan-Yeung, M; Bernstein, D I (2013). Asthma in the Workplace (4, illustrated, revised ed.). CRC Press. p. 198. ISBN 978-1-842-14591-3.
- ^ Gerald, Lynn B; Gerald, Joe K; McClure, Leslie A; Harrington, Kathy; Erwin, Sue; Bailey, William C (2011). "Redesigning a large school-based clinical trial in response to changes in community practice". Clinical Trials: Journal of the Society for Clinical Trials. 8 (3): 311–319. doi:10.1177/1740774511403513. ISSN 1740-7745. PMC 3145214. PMID 21730079.
- ^ "RTECS BO3150000 Ammonium, alkyldimethylbenzyl - , chloride". 28 March 2018.
- ^ Lewis R J Sr (2004). Lewis, Richard J (ed.). Sax's Dangerous Properties of Industrial Materials (11 ed.). Wiley-Interscience, Wiley & Sons, Inc. Hoboken, NJ. p. 104. doi:10.1002/0471701343. ISBN 978-0471701347.
SAFETY PROFILE: Poison by ingestion. Moderately toxic by skin contact. A severe eye irritant. A bactericide and fungicide. Dangerous; when heated to decomposition it emits toxic fumes of Cl- and NOx.
- ^ "TOXNET Benzalkonium Chloride Compounds".
- ^ "Haz-Map Benzalkonium Chloride". Archived from the original on 2014-10-06. Retrieved 2014-11-11.
- ^ "NIOSH ICSC Benzalkonium Chloride". Archived from the original on 2017-11-16. Retrieved 2017-09-08.
- ^ Seymour Stanton Block (2001). Disinfection, sterilization, and preservation (5, illustrated ed.). Lippincott Williams & Wilkins. p. 311. ISBN 978-0-683-30740-5.
- ^ Dart, R C (2004). Medical Toxicology (illustrated, revised ed.). Lippincott Williams & Wilkins. p. 125. ISBN 978-0-781-72845-4.
- ^ Gossel, T A (1994). Principles Of Clinical Toxicology, Third Edition (3, illustrated, revised ed.). CRC Press. ISBN 9780781701259.
- ^ Adelstein, Jake (2018-09-01). "Examining the motives behind mass murder in Japan". The Japan Times. Retrieved 2020-08-04.
- ^ Ryall, Julian (2018-07-10). "Japanese nurse investigated over 20 killings at end of shifts to avoid 'nuisance' of telling families of deaths". The Telegraph. ISSN 0307-1235. Retrieved 2018-08-26.
- ^ Campbell, A; Chapman, M (2008). Handbook of Poisoning in Dogs and Cats. John Wiley & Sons. p. 17. ISBN 978-0-470-69844-0.
- ^ García, MR; Cabo, ML (June 2018). "Optimization of E. coli Inactivation by Benzalkonium Chloride Reveals the Importance of Quantifying the Inoculum Effect on Chemical Disinfection". Frontiers in Microbiology. 9: 1259. doi:10.3389/fmicb.2018.01259. PMC 6028699. PMID 29997577.
- ^
 Dyer, David L.; Gerenratch, Kenneth B.; Wadhams, Peter S. (1998). "Testing a New Alcohol-Free Hand Sanitizer to Combat Infection". AORN Journal. 68 (2): 239–251. doi:10.1016/s0001-2092(06)62517-9. ISSN 0001-2092. PMID 9706236.
Dyer, David L.; Gerenratch, Kenneth B.; Wadhams, Peter S. (1998). "Testing a New Alcohol-Free Hand Sanitizer to Combat Infection". AORN Journal. 68 (2): 239–251. doi:10.1016/s0001-2092(06)62517-9. ISSN 0001-2092. PMID 9706236.
- ^ Jump up to: a b c d Rowe, Raymond (2009). Handbook of Pharmaceutical Excipients 6th Edition. London, UK: Pharmaceutical Press. pp. 56–58. ISBN 978-1-58212-135-2.
- ^ "FDA issues final rule on safety and effectiveness of consumer hand sanitizers". United States Food and Drug Administration. Retrieved 23 March 2020.
- ^ "Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use". 2016-09-06. Retrieved 2016-10-05.
Further reading[]
- Rieger, M M (1997). "The Skin Irritation Potential of Quaternaries" (PDF). Soc. Cosmet. Chem. 48: 307–317. Archived from the original (PDF) on 2014-12-07.
- Thorup I: Evaluation of health hazards by exposure to Quaternary ammonium compounds, The Institute of Food Safety and Toxicology, Danish Veterinary and Food Administration, [3]
- Verret, DJ; Marple, BF. (Feb 2005). "Effect of topical nasal steroid sprays on nasal mucosa and ciliary function". Curr Opin Otolaryngol Head Neck Surg. 13 (1): 14–8. doi:10.1097/00020840-200502000-00005. PMID 15654209. S2CID 33686959.
External links[]
- International Programme on Chemical Safety, International Chemical Safety Card (ICSC) - Benzalkonium Chloride
- National Institute for Occupational Safety and Health (NIOSH), International Chemical Safety Card (ICSC) - Benzalkonium Chloride
- International Programme on Chemical Safety, Poisons Information Monograph (PIMs) - Benzalkonium Chloride
- Haz-Map Category Details - Benzalkonium Chloride
- ToxNet Human Safety Database - Benzalkonium Chloride Compounds
- Recognition and Management of Pesticide Poisonings, United States Environmental Protection Agency, Office of Pesticide Programs, Sixth Edition, 2013
- CDC Healthcare Infection Control Practices Advisory Committee (HICPAC), Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008
- Santa Cruz Biotechnology, Inc. MSDS
- Spectrum Labs "Clear Bath" Algae Inhibitor MSDS
- Nile Chemicals MSDS
- TCI America MSDS
- Sciencelab.com, Inc. MSDS
- Nasal Saline Sprays - The Additives May Be the Problem
- Algaecides
- Antiseptics
- Benzyl compounds
- Cationic surfactants
- Chlorides
- Quaternary ammonium compounds