Pauson–Khand reaction

The Pauson–Khand reaction (or PKR or PK-type reaction) is a chemical reaction described as a [2+2+1] cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone.[1][2] The reaction was discovered by Ihsan Ullah Khand (1935-1980), who was working as a postdoctoral associate with Peter Ludwig Pauson (1925–2013)[3] at the University of Strathclyde in Glasgow. The seminal report dates back to 1970, however a detailed follow up was reported in 1973.[4][5] Initial findings by Pauson and Khand were intermolecular in nature, however many intramolecular examples have been highlighted in both synthesis and methodology reports, starting a decade later from reaction discovery.[6] This reaction was originally mediated by stoichiometric amounts of dicobalt octacarbonyl, but newer versions are both more efficient and catalytic utilizing different chiral auxiliaries for stereo induction, main group transition-metals (Ti, Mo, W, Fe, Co, Ni, Ru, Rh, Ir and Pd), and additives to enhance rate of reactivity and yield.[7][8][9][10] For a more extensive review on PKR, refer to Torres' book.[8]
Mechanism[]
While the mechanism isn't fully elucidated, seminal work proposed by Magnus in the 1985 is widely accepted for both mono and dinuclear catalysts.[11][12] Furthermore, computational studies published by Nakamura and Yamanaka in 2001 corroborate the initially proposed mechanism.[13] The mechanism is initiated by complexation between an alkene, alkyne and low valent metal to form a metallacyclopentene complex. Thereafter, CO inserts into one of the M-C bonds via migratory insertion, followed by reductive elimination to deliver the cyclopentenone. In the graphical depiction: 1. Alkyne Coordination, Insertion and Ligand Dissociation to form an 18-electron complex; 2. Ligand Dissociation forming a 16-electron complex; 3. Alkene Coordination to form an 18-electron complex; 4. Alkene Insertion and Ligand Association (synperiplanar, still 18-electron complex); 5. CO migratory insertion 6,7; Reductive Elimination of metal (loss of [Co2(CO)6]; 8. CO association, to regenerate the active organometallic complex.[14] Typically, the dissociation of carbon monoxide from the organometallic complex is rate limiting.[7]

Selectivity[]
With unsymmetrical alkenes or alkynes, regioselectivity can be problematic, but less so with intramolecular reactions.[15]
The reaction works with both terminal and internal alkynes although internal alkynes tend to give lower yields. The order of reactivity for the alkene is strained cyclic alkene > terminal alkene > disubstituted alkene > trisubstituted alkene. Unsuitable alkenes are tetrasubstituted alkenes and alkenes with strongly electron withdrawing groups.[16]

Mono substituted alkenes are generally directed by the sterics of the alkyne. For intermolecular PKR, the sterically larger group on the alkyne tends to go in the C2-position. Electron withdrawing groups on the alkyne prefer the C3-position.[17]
Mono substituted alkenes are difficult to discriminate between the C4- and C5-position, unless C2-position is sterically congested, or unless there is a chelating heteroatom on the alkene.[18][19]

Intramolecular PKR has been widely exploited in total synthesis, and is amenable to the formation of bicyclic rings (specifically 5,5- and 6,5- fused bicyclic rings), as well as more general polycyclic ring structures.[8] Generally, the reaction is highly syn selective about the bridgehead hydrogen, and substituents on the cyclopentane.

Catalysis[]
The PKR can be rendered catalytic. Buchwald showed that titanium[20][21] and nickel[22] complexes are amenable to this transformation. Additionally, Negishi found that zirconium[23][24] worked as well. Other metals can also be employed in these transformations.[25][8]

Traditional catalytic aids such as phosphine ligands can make the cobalt complex too stable, however bulky phosphite ligands are operable.[26] These reactions can be made enantioselective as well, with the addition of chiral ligands or auxiliaries. BINAP is commonly employed.[27] Extensive information can be found in a book written by Jacobsen.[28]
Additives[]
Typical PKR conditions are at elevated temperatures and pressures in aromatic/hydrocarbon (benzene, toluene) or ethererate (tetrahydrofuran, 1,2-dichloroethane) based solvents. These harsh conditions, may be attenuated with the addition of various additives. Many different tactics have been exploited to ameliorate these harsh conditions, making it amenable for additional functional groups, granting higher yields, shorter reaction times, and typically greater stereocontrol. Some of the different approaches are: adsorption, amine N-oxides and hydrates, and the addition of lewis basic molecules.
Adsorption[]
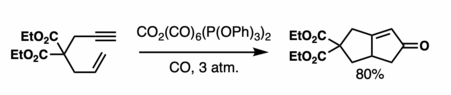
Adsorbing the metallic complex onto silica or alumina can enhance the rate of decarbonylative ligand exchange as exhibited in the image below.[29][30] This is because the donor posits itself on a solid surface (i.e. silica). Additionally using a solid support restricts conformational movement (rotamer effect).[31][32][33]

Amine N-Oxides and Hydrates[]
The two most common Amine N-Oxides are N-Methylmorpholine N-oxide (NMO) and Trimethylamine N-oxide (TMANO). It is believed that these additives remove carbon monoxide ligands via nucleophilic attack of the N-oxide onto the CO carbonyl, oxidizing the CO into CO2, and generating an unsaturated organometallic complex.[34][35] As a result, this renders the first step of the mechanism irreversible, and allows for more mild conditions. Hydrates of the aforementioned amine n-oxides have also been utilized.[36][37][38]

Schreiber utilized an Amine N-Oxide additive for the total synthesis of epoxydictymene.[39] The N-Oxide additive, while lower yielding, gave a d.r. of 11:1 in favor of the desired diastereomer (indicated at the red hydrogen), where as other conditions like thermal and ultrasonic were less effective.

Lewis Basic Additives[]
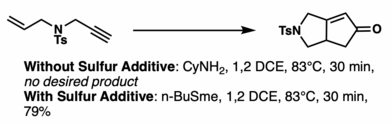
The most common lewis basic additive is sulfur containing compounds like n-BuSMe. Lewis basic additives are believed to accelerate the decarbonylative ligand exchange process. However, an alternative view posited in 2005, claims that the lewis basicity makes olefin insertion an irreversible step in the mechanistic pathway.[40] However, these sulfur adducts are typically hard to handle and smelly, so recently, n-dodecyl methyl sulfide (DodSMe) has been utilized as an alternative lewis basic source.[41] Another example is with TMTU (tetramethylthiourea).[42] Additionally, Chiral N-oxides have been used for enantio induction.[43][44]
Miscellaneous[]
Gas Free PKR[]
Recently, several groups have published work avoiding the use of toxic carbon monoxide, and instead generate the cyclopentenone carbonyl motif from aldehydes, carboxylic acids, and formates.[45][46][47] These examples typically employ Rhodium as the organometallic transition metal, as it is commonly used in decarbonylation reactions. The decarbonylation and PKR occur in the same reaction vessel.
Stable Cobalt Complexes[]
Canonical PKR utilizes low-valent Cobalt, however, these complexes are typically unstable, and efforts have been focused on preparing low-valent cobalt from bench (and generally) more stable cobalt complexes. Chung reported a catalytic PKR utilizing Co(acac)2, where upon exposure to sodium borohydride (NaBH4), generates the active catalytic species.[48]
Multinuclear Cobalt Catalysts[]
Generally, multinuclear cobalt catalysts like (Co)4(CO)12 and Co3(CO)9(μ3-CH) have been explored, however these complexes readily undergo oligomerization and often require harsh conditions.[49][50]
Takayama and co-workers used an intramolecular Pauson–Khand reaction to cyclise an enyne containing a tert-butyldiphenylsilyl (TBDPS) protected primary alcohol.[51] This was a key step in the asymmetric total synthesis of the Lycopodium alkaloid . The inclusion of a cyclic siloxane moiety in the reagent ensures that the product is formed with the desired conformation[52] – only a single enantiomer of the product was obtained in the Pauson–Khand reaction sequence.[51]

Variations[]
Wilkinson's catalyst, based on the transition metal rhodium, also effectively catalyses PK reactions but requires silver triflate as a co-catalyst.[53]

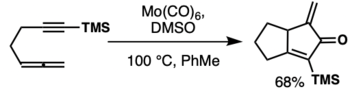
Molybdenum hexacarbonyl is a carbon monoxide donor in PK-type reactions between allenes and alkynes with dimethyl sulfoxide in toluene.[54] In general allenes can work in PKR. One example below is with molybdenum, however rhodium gives different regioselectivity. DFT investigations show the differences in selectivity are due to the different transition state metal geometries.[55]
The total synthesis of physostigmine by the Mukai group utilized the PKR on a carbodiimide.[56]

Cyclobutadiene also lends itself to a [2+2+1] cycloaddition although this reactant is generated in situ from decomplexation of stable cyclobutadiene iron tricarbonyl with ceric ammonium nitrate (CAN).[57]

An example of a newer version is the use of the chlorodicarbonylrhodium(I) dimer, [(CO)2RhCl]2, in the synthesis of (+)-phorbol by Phil Baran. In addition to using a rhodium catalyst, this synthesis features an intramolecular cyclization that results in the normal 5-membered α,β-cyclopentenone as well as 7-membered ring.[58]
See also[]
References[]
- ^ Pauson, P. L.; Khand, I. U. (1977). "Uses of Cobalt-Carbonyl Acetylene Complexes in Organic Synthesis". Ann. N. Y. Acad. Sci. 295 (1): 2–14. Bibcode:1977NYASA.295....2P. doi:10.1111/j.1749-6632.1977.tb41819.x. S2CID 84203764.
- ^ Blanco-Urgoiti, Jaime; Añorbe, Loreto; Pérez-Serrano, Leticia; Domínguez, Gema; Pérez-Castells, Javier (2004). "The Pauson–Khand reaction, a powerful synthetic tool for the synthesis of complex molecules". Chem. Soc. Rev. 33 (1): 32–42. doi:10.1039/b300976a. PMID 14737507.
- ^ Werner, Helmut (2014). "Obituary: Peter Ludwig Pauson (1925–2013)". Angew. Chem. Int. Ed. 53 (13): 3309. doi:10.1002/anie.201400432.
- ^ Khand, Ihsan U.; Knox, Graham R.; Pauson, Peter L.; Watts, William E. (1973). "Organocobalt complexes. Part I. Arene complexes derived from dodecacarbonyltetracobalt". Journal of the Chemical Society, Perkin Transactions 1: 975–977. doi:10.1039/p19730000975. ISSN 0300-922X.
- ^ Khand, Ihsan U.; Knox, Graham R.; Pauson, Peter L.; Watts, William E.; Foreman, Michael I. (1973). "Organocobalt complexes. Part II. Reaction of acetylenehexacarbonyldicobalt complexes, (R1C2R2)Co2(CO)6, with norbornene and its derivatives". Journal of the Chemical Society, Perkin Transactions 1: 977–981. doi:10.1039/p19730000977. ISSN 0300-922X.
- ^ Schore, N. E.; Croudace, M. C. (1981-12-01). "Preparation of bicyclo[3.3.0]oct-1-en-3-one and bicyclo[4.3.0]non-1(9)-en-8-one via intramolecular cyclization of .alpha.,.omega.-enynes". The Journal of Organic Chemistry. 46 (26): 5436–5438. doi:10.1021/jo00339a046. ISSN 0022-3263.
- ^ a b Hartwig, John F. (2010). Organotransition metal chemistry : from bonding to catalysis. Mill Valley, Calif. ISBN 978-1-891389-53-5. OCLC 310401036.
- ^ a b c d Ramón Ríos Torres (2012). The Pauson-Khand reaction : scope, variations, and applications. Hoboken, N.J.: John Wiley & Sons. ISBN 978-1-118-30863-9. OCLC 774982574.
- ^ Schore, Neil E. (1991). "The Pauson–Khand Cycloaddition Reaction for Synthesis of Cyclopentenones". Org. React. 40: 1–90. doi:10.1002/0471264180.or040.01. ISBN 0471264180.
- ^ Gibson, Susan E.; Stevenazzi, Andrea (2003). "The Pauson–Khand Reaction: The Catalytic Age Is Here!". Angew. Chem. Int. Ed. 42 (16): 1800–1810. doi:10.1002/anie.200200547. PMID 12722067.
- ^ Magnus, Philip; Exon, Christopher; Albaugh-Robertson, Pamela (1985-01-01). "Dicobaltoctacarbonyl-alkyne complexes as intermediates in the synthesis of bicyclo[3.3.0]octenones for the synthesis of coriolin and hirsutic acid". Tetrahedron. 41 (24): 5861–5869. doi:10.1016/S0040-4020(01)91425-5. ISSN 0040-4020.
- ^ Magnus, Philip; Principe, Lawrence M. (January 1985). "Origins of 1,2- and 1,3-stereoselectivity in dicobaltoctacarbonyl alkene-alkyne cyclizations for the synthesis of substituted bicyclo[3.3.0]octenones". Tetrahedron Letters. 26 (40): 4851–4854. doi:10.1016/s0040-4039(00)94968-2. ISSN 0040-4039.
- ^ Yamanaka, Masahiro; Nakamura, Eiichi (2001-02-01). "Density Functional Studies on the Pauson−Khand Reaction". Journal of the American Chemical Society. 123 (8): 1703–1708. doi:10.1021/ja005565+. ISSN 0002-7863. PMID 11456770.
- ^ Kürti, László (2005). Strategic applications of named reactions in organic synthesis : background and detailed mechanisms. Barbara Czakó. Amsterdam: Elsevier Academic Press. ISBN 978-0-12-429785-2. OCLC 60792519.
- ^ Jeong, Nakcheol; Hwang, Sung Hee; Lee, Youngshin; Chung, Young Keun (1994). "Catalytic version of the Intramolecular Pauson-Khand Reaction". Journal of the American Chemical Society. 116 (7): 3159. doi:10.1021/ja00086a070.
- ^ Strategic applications of named reactions in organic synthesis: background and details mechanisms 2007 László Kürti,Barbara Czakó
- ^ Robert, Frédéric; Milet, Anne; Gimbert, Yves; Konya, Denés; Greene, Andrew E. (2001-06-01). "Regiochemistry in the Pauson−Khand Reaction: Has a Trans Effect Been Overlooked?". Journal of the American Chemical Society. 123 (23): 5396–5400. doi:10.1021/ja004170n. ISSN 0002-7863. PMID 11389617.
- ^ Exon, Christopher; Magnus, Philip (1983-04-01). "Stereoselectivity of intramolecular dicobalt octacarbonyl alkene-alkyne cyclizations: short synthesis of dl-coriolin". Journal of the American Chemical Society. 105 (8): 2477–2478. doi:10.1021/ja00346a063. ISSN 0002-7863.
- ^ Krafft, Marie E. (1988-02-03). "Regiocontrol in the intermolecular cobalt-catalyzed olefin-acetylene cycloaddition". Journal of the American Chemical Society. 110 (3): 968–970. doi:10.1021/ja00211a048. ISSN 0002-7863.
- ^ Hicks, Frederick A.; Buchwald, Stephen L. (1996-01-01). "Highly Enantioselective Catalytic Pauson−Khand Type Formation of Bicyclic Cyclopentenones". Journal of the American Chemical Society. 118 (46): 11688–11689. doi:10.1021/ja9630452. ISSN 0002-7863.
- ^ Hicks, Frederick A.; Kablaoui, Natasha M.; Buchwald, Stephen L. (January 1996). "Titanocene-Catalyzed Cyclocarbonylation of Enynes to Cyclopentenones". Journal of the American Chemical Society. 118 (39): 9450–9451. doi:10.1021/ja9621509. ISSN 0002-7863.
- ^ Zhang, Minghui; Buchwald, Stephen L. (January 1996). "A Nickel(0)-Catalyzed Process for the Transformation of Enynes to Bicyclic Cyclopentenones". The Journal of Organic Chemistry. 61 (14): 4498–4499. doi:10.1021/jo960410z. ISSN 0022-3263. PMID 11667365.
- ^ Negishi, Eiichi; Holmes, Steven J.; Tour, James M.; Miller, Joseph A. (1985-04-01). "Metal promoted cyclization. 7. Zirconium-promoted bicyclization of enynes". Journal of the American Chemical Society. 107 (8): 2568–2569. doi:10.1021/ja00294a071. ISSN 0002-7863.
- ^ Negishi, Eiichi; Holmes, Steven J.; Tour, James M.; Miller, Joseph A.; Cederbaum, Fredrik E.; Swanson, Douglas R.; Takahashi, Tamotsu (April 1989). "Metal-promoted cyclization. 19. Novel bicyclization of enynes and diynes promoted by zirconocene derivatives and conversion of zirconabicycles into bicyclic enones via carbonylation". Journal of the American Chemical Society. 111 (9): 3336–3346. doi:10.1021/ja00191a035. ISSN 0002-7863.
- ^ Jeong, Nakcheol; Hwang, Sung Hee; Lee, Youngshin; Chung, Young Keun (April 1994). "Catalytic version of the Intramolecular Pauson-Khand Reaction". Journal of the American Chemical Society. 116 (7): 3159–3160. doi:10.1021/ja00086a070. ISSN 0002-7863.
- ^ Jeong, Nakcheol; Hwang, Sung Hee; Lee, Youngshin; Chung, Young Keun (April 1994). "Catalytic version of the Intramolecular Pauson-Khand Reaction". Journal of the American Chemical Society. 116 (7): 3159–3160. doi:10.1021/ja00086a070. ISSN 0002-7863.
- ^ Jeong, Nakcheol; Sung, Byung Kee; Choi, Yoon Kyung (2000-07-01). "Rhodium(I)-Catalyzed Asymmetric Intramolecular Pauson−Khand-Type Reaction". Journal of the American Chemical Society. 122 (28): 6771–6772. doi:10.1021/ja0007049. ISSN 0002-7863.
- ^ Jacobsen, Eric N. (1999), "Future Perspectives in Asymmetric Catalysis", Comprehensive Asymmetric Catalysis I–III, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 1473–1477, doi:10.1007/978-3-642-58571-5_24, ISBN 978-3-642-63651-6, retrieved 2021-06-15
- ^ Billington, David C.; Willison, Debra (1984). "A simple organocobalt mediated synthesis of substituted 3-oxabicyclo[3.3.0]oct-6-en-7-ones". Tetrahedron Letters. 25 (36): 4041–4044. doi:10.1016/0040-4039(84)80061-1. ISSN 0040-4039.
- ^ Morimoto, Takashi; Hirano, Masao; Echigoya, Kohki; Sato, Takafumi (1986). "Oxidation by cobalt(III) acetate. Part 10. Effects of ring substituents on the product distributions in the oxidation of β-methylstyrenes by cobalt(III) acetate in acetic acid". J. Chem. Soc., Perkin Trans. 2 (8): 1205–1209. doi:10.1039/p29860001205. ISSN 0300-9580.
- ^ Brown, Scott W.; Pauson, Peter L. (1990). "The synthesis of nitrogen heterocycles via the intramolecular Khand reaction: formation of tetra- and hexa-hydrocyclopenta[c]pyrrol-5(1H)-ones and hexahydro-6H-2-pyrindin-6-ones". Journal of the Chemical Society, Perkin Transactions 1 (4): 1205–1209. doi:10.1039/p19900001205. ISSN 0300-922X.
- ^ Shilov, Aleksandr E; Shul'pin, Georgiy B (1987-05-31). "Activation and Catalytic Reactions of Alkanes in Solutions of Metal Complexes". Russian Chemical Reviews. 56 (5): 442–464. Bibcode:1987RuCRv..56..442S. doi:10.1070/rc1987v056n05abeh003282. ISSN 0036-021X.
- ^ Smit, W.A; Kireev, S.L; Nefedov, O.M; Tarasov, V.A (1989-01-01). "Methylenecyclopropane as an alkene component in the Khand-Pauson reaction". Tetrahedron Letters. 30 (30): 4021–4024. doi:10.1016/S0040-4039(00)99313-4. ISSN 0040-4039.
- ^ Shambayani, Soroosh; Crowe, William E.; Schreiber, Stuart L. (1990-01-01). "N-oxide promoted pauson-khand cyclizations at room temperature". Tetrahedron Letters. 31 (37): 5289–5292. doi:10.1016/S0040-4039(00)98052-3. ISSN 0040-4039.
- ^ Alper, Howard; Edward, J. T. (2011-02-03). "Reactions of iron pentacarbonyl with compounds containing the N—O linkage". Canadian Journal of Chemistry. 48 (10): 1543–1549. doi:10.1139/v70-251.
- ^ Crawford, James J.; Kerr, William J.; McLaughlin, Mark; Morrison, Angus J.; Pauson, Peter L.; Thurston, Graeme J. (2006-12-04). "Use of a highly effective intramolecular Pauson–Khand cyclisation for the formal total synthesis of (±)-α- and β-cedrene by preparation of cedrone". Tetrahedron. 62 (49): 11360–11370. doi:10.1016/j.tet.2006.05.044. ISSN 0040-4020.
- ^ Krafft, Marie E.; Romero, Romulo H.; Scott, Ian L. (1992-09-01). "Pauson-Khand reaction with electron-deficient alkynes". The Journal of Organic Chemistry. 57 (20): 5277–5278. doi:10.1021/jo00046a001. ISSN 0022-3263.
- ^ Bernardes, Vania; Kann, Nina; Riera, Antoni; Moyano, Albert; Pericas, Miquel A.; Greene, Andrew E. (1995-10-01). "Asymmetric Pauson-Khand Cyclization: A Formal Total Synthesis of Natural Brefeldin A". The Journal of Organic Chemistry. 60 (21): 6670–6671. doi:10.1021/jo00126a010. ISSN 0022-3263.
- ^ Jamison, Timothy F.; Shambayati, Soroosh; Crowe, William E.; Schreiber, Stuart L. (1997-05-01). "Tandem Use of Cobalt-Mediated Reactions to Synthesize (+)-Epoxydictymene, a Diterpene Containing a Trans-Fused 5−5 Ring System". Journal of the American Chemical Society. 119 (19): 4353–4363. doi:10.1021/ja970022u. ISSN 0002-7863.
- ^ Valle, Carlos Perez del; Milet, Anne; Gimbert, Yves; Greene, Andrew E. (2005). "Lewis Base Promoters in the Pauson–Khand Reaction: A Different Scenario". Angewandte Chemie International Edition. 44 (35): 5717–5719. doi:10.1002/anie.200500955. ISSN 1521-3773. PMID 16078280.
- ^ Cochrane, Alison R.; Kerr, William J.; Paterson, Laura C.; Pearson, Colin M.; Shaw, Paul (2021-01-08). "Advances in the cobalt-catalysed Pauson-Khand reaction: Development of a sulfide-promoted, microwave-assisted protocol". Tetrahedron. 78: 131805. doi:10.1016/j.tet.2020.131805. ISSN 0040-4020. S2CID 229387356.
- ^ Reuter, Carin; Vögtle, Fritz (March 2000). "Rotaxanes via Michael Addition†". Organic Letters. 2 (5): 593–595. doi:10.1021/ol990350u. ISSN 1523-7060. PMID 10814386.
- ^ Carbery, David R.; Kerr, William J.; Lindsay, David M.; Scott, James S.; Watson, Stephen P. (2000-04-22). "Preparation and reaction of desymmetrised cobalt alkyne complexes". Tetrahedron Letters. 41 (17): 3235–3239. doi:10.1016/S0040-4039(00)00356-7. ISSN 0040-4039.
- ^ Jończyk, Andrzej; Konarska, Anna (July 1999). "Generation and Reactions of Ammonium Ylides in Basic Two-Phase Systems: Convenient Synthesis of Cyclopropanes, Oxiranes and Alkenes Substituted with Electron-Withdrawing Groups". Synlett. 1999 (7): 1085–1087. doi:10.1055/s-1999-2757. ISSN 0936-5214.
- ^ Morimoto, Tsumoru; Fuji, Koji; Tsutsumi, Ken; Kakiuchi, Kiyomi (2002-04-01). "CO-Transfer Carbonylation Reactions. A Catalytic Pauson−Khand-Type Reaction of Enynes with Aldehydes as a Source of Carbon Monoxide". Journal of the American Chemical Society. 124 (15): 3806–3807. doi:10.1021/ja0126881. ISSN 0002-7863. PMID 11942798.
- ^ Shibata, Takanori; Toshida, Natsuko; Takagi, Kentaro (2002-05-01). "Catalytic Pauson−Khand-Type Reaction Using Aldehydes as a CO Source". Organic Letters. 4 (9): 1619–1621. doi:10.1021/ol025836g. ISSN 1523-7060. PMID 11975643.
- ^ "Front cover". Chemical Communications (25): 2545–2546. 2007. doi:10.1039/b708443a. ISSN 1359-7345.
- ^ Lee, Nam Young; Chung, Young Keun (April 1996). "Synthesis of cyclopentenones: The new catalytic cocyclization reaction of alkyne, alkene, and carbon monoxide employing catalytic Co(acac)2 and NaBH4". Tetrahedron Letters. 37 (18): 3145–3148. doi:10.1016/0040-4039(96)00513-8. ISSN 0040-4039.
- ^ Kim, Jong Wook; Chung, Young Keun (February 1998). "Pauson-Khand Reaction Catalyzed by Co4(CO)12". Synthesis. 1998 (2): 142–144. doi:10.1055/s-1998-2016. ISSN 0039-7881.
- ^ Sugihara, Takumichi; Yamaguchi, Masahiko (1998-10-01). "The Pauson−Khand Reaction Catalyzed by the Methylidynetricobalt Nonacarbonyl Cluster". Journal of the American Chemical Society. 120 (41): 10782–10783. doi:10.1021/ja982635s. ISSN 0002-7863.
- ^ a b Nakayama, Atsushi; Kogure, Noriyuki; Kitajima, Mariko; Takayama, Hiromitsu (2011). "Asymmetric Total Synthesis of a Pentacyclic Lycopodium Alkaloid: Huperzine‐Q". Angew. Chem. Int. Ed. 50 (35): 8025–8028. doi:10.1002/anie.201103550. PMID 21751323.
- ^ Ho, Tse-Lok (2016). "Dicobalt Octacarbonyl". Fiesers' Reagents for Organic Synthesis. Vol. 28. John Wiley & Sons. pp. 251–252. ISBN 9781118942819.
- ^ Nakcheol Jeong, Byung Ki Sung, Jin Sung Kim, Soon Bong Park,Sung Deok Seo, Jin Young Shin, Kyu Yeol In, Yoon Kyung Choi Pauson–Khand-type reaction mediated by Rh(I) catalysts Pure Appl. Chem., Vol. 74, No. 1, pp. 85–91, 2002. (Online article)
- ^ Kent, J (1995). "A new allenic Pauson-Khand cycloaddition for the preparation of α-methylene cyclopentenones". Tetrahedron Letters. 36 (14): 2407–2410. doi:10.1016/0040-4039(95)00315-4.
- ^ Bayden, Alexander S.; Brummond, Kay M.; Jordan, Kenneth D. (2006-10-01). "Computational Insight Concerning Catalytic Decision Points of the Transition Metal Catalyzed [2 + 2 + 1] Cyclocarbonylation Reaction of Allenes". Organometallics. 25 (22): 5204–5206. doi:10.1021/om0607503. ISSN 0276-7333. PMC 4441411. PMID 26005240.
- ^ Mukai, Chisato; Yoshida, Tatsunori; Sorimachi, Mao; Odani, Akira (January 2006). "Co2(CO)8-Catalyzed Intramolecular Hetero-Pauson−Khand Reaction of Alkynecarbodiimide: Synthesis of (±)-Physostigmine". Organic Letters. 8 (1): 83–86. doi:10.1021/ol052562z. ISSN 1523-7060. PMID 16381573.
- ^ Intramolecular [2+2+1] Cycloadditions with (Cyclobutadiene)tricarbonyliron Benjamin A. Seigal, Mi Hyun An, Marc L. Snapper Angewandte Chemie International Edition Volume 44, Issue 31 , Pages 4929 - 4932 2005. doi:10.1002/anie.200501100
- ^ Kawamura, Shuhei; Chu, Hang; Felding, Jakob; Baran, Phil S. (2016). "Nineteen-step total synthesis of (+)-phorbol". Nature. 532 (7597): 90–93. Bibcode:2016Natur.532...90K. doi:10.1038/nature17153. PMC 4833603. PMID 27007853.
- Cycloadditions
- Multiple component reactions
- Name reactions