Potassium citrate
| |
| Names | |
|---|---|
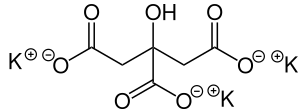
| Preferred IUPAC name
Tripotassium 2-hydroxypropane-1,2,3-tricarboxylate | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.596 |
| E number | E332(ii) (antioxidants, ...) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| K3C6H5O7 | |
| Molar mass | 306.395 g/mol |
| Appearance | white powder hygroscopic |
| Odor | odorless |
| Density | 1.98 g/cm3 |
| Melting point | 180 °C (356 °F; 453 K)[1] |
| Boiling point | 230 °C (446 °F; 503 K)[1] |
| soluble | |
| Solubility | soluble in glycerin insoluble in ethanol (95%) |
| Acidity (pKa) | 8.5 |
| Pharmacology | |
| A12BA02 (WHO) | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
170 mg/kg (IV, dog) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium citrate (also known as tripotassium citrate) is a potassium salt of citric acid with the molecular formula K3C6H5O7. It is a white, hygroscopic crystalline powder. It is odorless with a saline taste. It contains 38.28% potassium by mass. In the monohydrate form, it is highly hygroscopic and deliquescent.
As a food additive, potassium citrate is used to regulate acidity, and is known as E number E332. Medicinally, it may be used to control kidney stones derived from uric acid or cystine.
Synthesis[]
Potassium citrate can be synthesized by the neutralization of Citric acid which is achieved by the addition of Potassium bicarbonate, Potassium carbonate or Potassium hydroxide to it. The solution can then be filtered and the solvent can be evaporated till granulation.
Uses[]
Potassium citrate is rapidly absorbed when given by mouth, and is excreted in the urine.[2] Since it is an alkaline salt, it is effective in reducing the pain and frequency of urination when these are caused by highly acidic urine.[3] It is used for this purpose in dogs and cats, but is chiefly employed as a non-irritating diuretic.
Potassium citrate is an effective way to treat/manage gout[4] and arrhythmia,[medical citation needed] if the patient is hypokalemic.
It is widely used to treat urinary calculi (kidney stones), and is often used by patients with cystinuria.[medical citation needed] A study of 500 patients with recurrent stones found that it reduced the frequency of stones from 2 per year to 0.5 per year.[citation needed]
It is also used as an alkalizing agent in the treatment of mild urinary tract infections, such as cystitis.[5]
It is also used in many soft drinks as a buffering agent.[6]
Frequently used in an aqueous solution with other potassium salts, it is a wet chemical fire suppressant that is particularly useful against kitchen fires. Its alkaline pH encourages saponification to insulate the fuel from oxidizing air, and the endothermic dehydration reaction absorbs heat energy to reduce temperatures.[7][8]
Administration[]
Potassium citrate is usually administered by mouth in dilute aqueous solution, because of its somewhat caustic effect on the stomach lining, and the potential for other mild health hazards.
References[]
- ^ Jump up to: a b Environmental Health & Safety MSDS Number:P5675
- ^ Medscape on hypocitraturia
- ^ Potassium Citrate for Kidney Stones
- ^ Potassium Citrate
- ^ "Potassium citrate for cystitis". patient.info.
- ^ https://www.livestrong.com/article/314839-soft-drinks-with-potassium-citrate/
- ^ US Fire extinguishing composition and method for fire extinguishing 5945025A, James A. Cunningham
- ^ Xiaofang Wangy et al 2019 IOP Conf. Ser.: Mater. Sci. Eng. 490 022047
External links[]
- Tanner, G.A. "Potassium citrate improves renal function in rats with polycystic kidney disease". Journal of the American Society of Nephrology. Retrieved December 17, 2016.
- Food acidity regulators
- Citrates
- Potassium compounds
- E-number additives