Previtamin D3

| |
| Names | |
|---|---|
| IUPAC name
Previtamin D3
| |
| Preferred IUPAC name
(1S)-4-Methyl-3-[(1Z)-2-{(1R,3aR,7aR)-7a-methyl-1-[(2R)-6-methylheptan-2-yl]-2,3,3a,6,7,7a-hexahydro-1H-inden-4-yl}ethen-1-yl]cyclohex-3-en-1-ol | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.304 |
| EC Number |
|
| MeSH | Previtamin+D(3) |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H44O | |
| Molar mass | 384.648 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
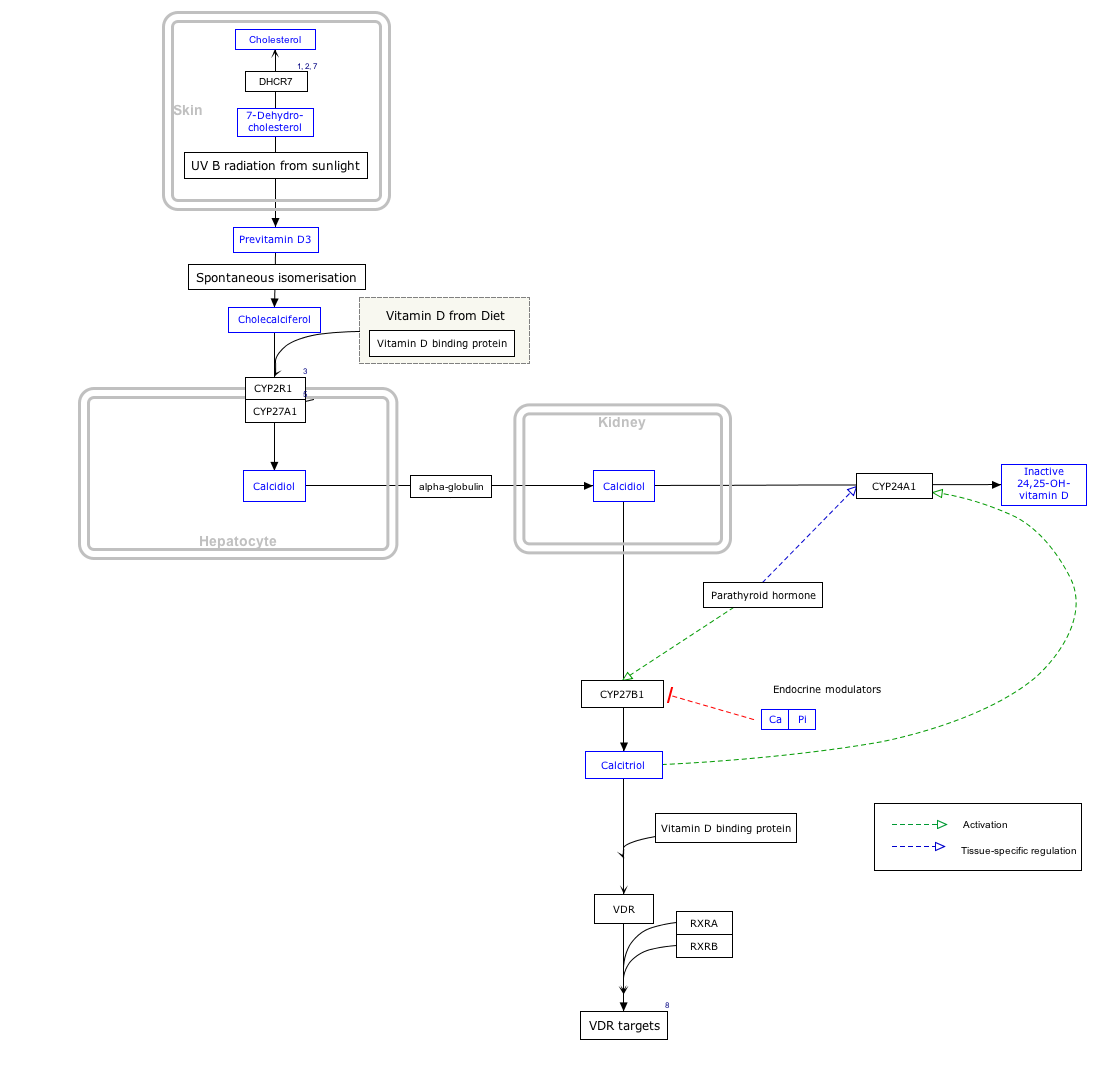
Previtamin D3 is an intermediate in the production of cholecalciferol (vitamin D3).
It is formed by the action of UV light, most specifically UVB light of wavelengths between 295 and 300 nm, acting on 7-dehydrocholesterol in the epidermal layers of the skin.[1][2][3]
The B ring of the steroid nucleus structure is broken open, making a secosteroid. This then undergoes spontaneous isomerization into cholecalciferol, the prohormone of the active form of vitamin D, calcitriol.
The synthesis of previtamin D3 is blocked effectively by sunscreens.[4]
Interactive pathway map[]
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "VitaminDSynthesis_WP1531".
References[]
- ^ MacLaughlin JA, Anderson RR, Holick MF (May 1982). "Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin". Science. 216 (4549): 1001–3. Bibcode:1982Sci...216.1001M. doi:10.1126/science.6281884. PMID 6281884.
- ^ Webb AR (September 2006). "Who, what, where and when-influences on cutaneous vitamin D synthesis". Progress in Biophysics and Molecular Biology. 92 (1): 17–25. doi:10.1016/j.pbiomolbio.2006.02.004. PMID 16766240.
- ^ Pope SJ, Holick MF, Mackin S, Godar DE (2008). "Action spectrum conversion factors that change erythemally weighted to previtamin D3-weighted UV doses". Photochemistry and Photobiology. 84 (5): 1277–83. doi:10.1111/j.1751-1097.2008.00373.x. PMID 18513232.
- ^ Sayre RM, Dowdy JC (2007). "Darkness at noon: sunscreens and vitamin D3". Photochemistry and Photobiology. 83 (2): 459–63. doi:10.1562/2006-06-29-RC-956. PMID 17115796.
Categories:
- Vitamin D
- Secosteroids
- Indanes
- Organic chemistry stubs