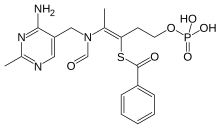
Benfotiamine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Milgamma |
| Other names | S-Benzoylthiamine O-monophosphate |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.906 |
| Chemical and physical data | |
| Formula | C19H23N4O6PS |
| Molar mass | 466.45 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Benfotiamine (rINN, or S-benzoylthiamine O-monophosphate) is a synthetic, fat-soluble, S-acyl derivative of thiamine (vitamin B1) that is approved in some countries as a medication or dietary supplement to treat diabetic sensorimotor polyneuropathy. Benfotiamine was developed in late 1950s in Japan.[1][2]
Uses[]
Benfotiamine is primarily marketed as an over-the-counter drug to treat diabetic polyneuropathy.[3] A 2021 review described two clinical trials and concluded that more research is needed.[4]
As of 2017, benfotiamine was marketed as a pharmaceutical drug in many countries under the following brand names: Benalgis, Benfogamma, Benforce, Benfotiamina, Biotamin, Biotowa, Milgamma, and Vilotram.[5] It was also marketed in some jurisdictions as a combination drug with cyanocobalamin as Milgamma, in combination with pyridoxine as Milgamma, in combination with metformin as Benforce-M, and with thiamine as Vitafos.[5]
Adverse effects[]
There is little published data on adverse effects. In one study of a combination of benfotiamine, pyridoxine, and cyanocobalamin, around 8% of people taking the drug experienced nausea, dizziness, stomach ache and weight gain.[6]
Pharmacology[]
Benfotiamine is dephosphorylated to S-benzoylthiamine by ecto-alkaline phosphatases present in the intestinal mucosa, and is then hydrolyzed to thiamine by thioesterases in the liver.[7] Benfotiamine is more bioavailable than thiamine salts,[8] providing higher levels of thiamine in muscle, brain, liver, and kidney.[6]
Benfotiamine mainly acts on peripheral tissues through an increase in transketolase activity.[7][6][9]
Chemistry[]
Benfotiamine is a lipid derivative of thiamine, specifically a synthetic S-acyl Vitamin B1 analogue; its chemical name is S-benzoylthiamine O-monophosphate.[10] It has very low solubility in water or other aqueous solvents.[7]
Research[]
Benfotiamine has been studied in laboratory models of diabetic retinopathy, neuropathy, and nephropathy.[10] A 2021 review described two clinical trials and concluded that more research is needed.[4]
Administration of benfotiamine may increase intracellular levels of thiamine diphosphate, a cofactor of transketolase,[10] and based on metabolic theories of Alzheimer's disease, it has been studied in preclinical models of Alzheimer's disease.[11][clarification needed]
References[]
- ^ Wada, T; Takagi, H; Minakami, H; Hamanaka, W; Okamoto, K; Ito, A; Sahashi, Y (21 July 1961). "A New Thiamine Derivative, S-Benzoylthiamine O-Monophosphate". Science. 134 (3473): 195–96. Bibcode:1961Sci...134..195W. doi:10.1126/science.134.3473.195. PMID 13782394. S2CID 10384617.
- ^ Sambon, Margaux; Wins, Pierre; Bettendorff, Lucien (2021-05-21). "Neuroprotective Effects of Thiamine and Precursors with Higher Bioavailability: Focus on Benfotiamine and Dibenzoylthiamine". International Journal of Molecular Sciences. 22 (11): 5418. doi:10.3390/ijms22115418. ISSN 1422-0067. PMC 8196556. PMID 34063830.
- ^ McCarty, MF; Inoguchi, T (2008). "11. Targeting Oxidant Stress as a Strategy for Preventing Vascular Complications of Diabetes and Metabolic Syndrome". In Pasupuleti, Vijai K.; Anderson, James W. (eds.). Nutraceuticals, glycemic health and type 2 diabetes (1st ed.). Ames, Iowa: Wiley-Blackwell/IFT Press. p. 213. ISBN 9780813804286.
- ^ a b Zaheer A, Zaheer F, Saeed H, Tahir Z, Tahir MW (April 2021). "A Review of Alternative Treatment Options in Diabetic Polyneuropathy". Cureus. 13 (4): e14600. doi:10.7759/cureus.14600. PMC 8139599. PMID 34040901.
- ^ a b "Benfotiamine International brands". Drugs.com. Retrieved 14 March 2017.
- ^ a b c Panel on Food Additives and Nutrient Sources added to Food (2008). "Scientific Opinion: Benfotiamine, thiamine monophosphate chloride and thiamine pyrophosphate chloride, as sources of vitamin B1 added for nutritional purposes to food supplements" (PDF). The EFSA Journal. 864: 1–31.
- ^ a b c Patel, SM; Patel, RP; Prajapati, BG (2012). Patel, S (ed.). "Solubility enhancement of benfotiamine, a lipid derivative of thiamine by solid dispersion technique". Journal of Pharmacy & Bioallied Sciences. US National Library of Medicine and National Institutes of Health: J Pharm Bioallied Sci. 4 (Suppl 1): S104–S105. doi:10.4103/0975-7406.94157. PMC 3467834. PMID 23066179.
- ^ Bitsch, R; Wolf, M; Moeller, J; Heuzeroth, L; Grueneklee, D (1991). "Bioavailability Assessment of the Lipophilic Benfotiamine as Compared to a Water-Soluble Thiamin Derivative". Ann Nutr Metab. 35 (5): 292–96. doi:10.1159/000177659. PMID 1776825.
- ^ Yamazaki, M (1968). "Studies on the absorption of S-benzoylthiamine O-monophosphate : (I) Metabolism in tissue homogenates". Vitamins. 38 (1): 12–20.
- ^ a b c Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A (June 2010). "The multifaceted therapeutic potential of benfotiamine". Pharmacol Res. 61 (6): 482–88. doi:10.1016/j.phrs.2010.02.008. PMID 20188835.
- ^ Gibson, GE; Hirsch, JA; Cirio, RT; Jordan, BD; Fonzetti, P; Elder, J (July 2013). "Abnormal thiamine-dependent processes in Alzheimer's Disease. Lessons from diabetes". Molecular and Cellular Neurosciences. 55: 17–25. doi:10.1016/j.mcn.2012.09.001. PMC 3609887. PMID 22982063.
- Aminopyrimidines
- Nitriles
- Organophosphates
- Thioesters
- Thiamine
- Formamides