Gabapentin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Neurontin, others[1] |
| Other names | CI-945; GOE-3450; DM-1796 (Gralise) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a694007 |
| License data |
|
| Pregnancy category |
|
| Dependence liability | Low |
| Addiction liability | Low |
| Routes of administration | By mouth |
| Drug class | Gabapentinoid |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 27–60% (inversely proportional to dose; a high fat meal also increases bioavailability)[3][4] |
| Protein binding | Less than 3%[3][4] |
| Metabolism | Not significantly metabolized[3][4] |
| Elimination half-life | 5 to 7 hours[3][4] |
| Excretion | Renal[3][4] |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.056.415 |
| Chemical and physical data | |
| Formula | C9H17NO2 |
| Molar mass | 171.240 g·mol−1 |
| 3D model (JSmol) | |
show
SMILES | |
show
InChI | |
Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat partial seizures and neuropathic pain.[5][6] It is a first-line medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain.[7] It is moderately effective: about 30-40% of those given gabapentin for diabetic neuropathy or postherpetic neuralgia have a meaningful benefit.[8]
Sleepiness and dizziness are the most common side effects. Serious side effects include an increased risk of suicide, respiratory depression, and allergic reactions.[5] Lower doses are recommended in those with kidney disease.[5] Gabapentin acts by decreasing activity of a subset of calcium channels.[9][10][11]
Gabapentin was first approved for use in 1993.[12] It has been available as a generic medication in the United States since 2004.[13] In 2018, it was the eleventh most commonly prescribed medication in the United States, with more than 45 million prescriptions.[14][15] During the 1990s, Parke-Davis, a subsidiary of Pfizer, used a number of illegal techniques to encourage physicians in the United States to prescribe gabapentin for unapproved uses.[16] They have paid out millions of dollars to settle lawsuits regarding these activities.[17]
Medical uses[]
Gabapentin is recommended for use in focal seizures and neuropathic pain.[5][6] Gabapentin is widely prescribed off-label in the US and UK,[18][19] for example, for the treatment of non-neuropathic pain,[18] anxiety disorders and bipolar disorder.[20]
Seizures[]
Gabapentin is approved for the treatment of focal seizures;[21] however, it is not effective for generalized epilepsy.[22]
Neuropathic pain[]
Gabapentin is recommended as a first-line treatment for chronic neuropathic pain by various medical authorities.[6][7][23][24] This is a general recommendation applicable to all neuropathic pain syndromes except for trigeminal neuralgia.[7][24]
In regard to the specific diagnoses, the best evidence exists for gabapentin treatment of postherpetic neuralgia and diabetic neuropathy.[8] Gabapentin is approved for the former indication in the US.[5] In addition to these two neuropathies, European Federation of Neurological Societies guideline notes gabapentin effectiveness for central pain.[7] A combination of gabapentin with an opioid or nortriptyline may work better than either drug alone.[7][24]
Overall, gabapentin shows moderate effectiveness for neuropathic pain. Only a minority of patients obtain meaningful relief. Out of 10 persons treated with gabapentin, three to four benefit substantially as compared to one to two persons treated with placebo.[8]
Evidence finds little or no benefit and significant risk in those with chronic low back pain or sciatica.[25][26] Gabapentin is not effective in HIV-associated sensory neuropathy[27] and neuropathic pain due to cancer.[28]
Anxiety[]
There is a paucity of research on the use of gabapentin for the treatment of anxiety disorders.[29][30] In a controlled trial of breast cancer survivors with anxiety,[30] and in a trial for social phobia,[29] gabapentin significantly reduced anxiety levels. For panic disorder, gabapentin is ineffective.[30][29] On the other hand, some psychiatric textbooks see "a possible role for gabapentin in anxiety disorders, particularly social phobia and panic disorder" based on "case reports and double-blind studies"[31] or state that controlled studies have not shown gabapentin to be very effective for psychiatric indications; "however, clinically it is effective".[20]
Drug dependence[]
Gabapentin is moderately effective in reducing the symptoms of alcohol withdrawal and associated craving.[32][33][34] The evidence in favor of gabapentin is weak in the treatment of alcoholism: it does not contribute to the achievement of abstinence, and the data on the relapse of heavy drinking and percent of days abstinent do not robustly favor gabapentin; it only decreases the percent days of heavy drinking.[35]
Gabapentin is ineffective in cocaine dependence and methamphetamine use,[36] and it does not increase the rate of smoking cessation.[37] Gabapentin does not significantly reduce the symptoms of opiate withdrawal.[36] There is insufficient evidence for its use in cannabis dependence.[38]
Other[]
Gabapentin is recommended as a first-line treatment of the acquired pendular nystagmus, torsional nystagmus, and infantile nystagmus; however, it does not work in periodic alternating nystagmus.[39][40][41]
Gabapentin decreases the frequency of hot flashes in both menopausal women and patients with breast cancer. However, antidepressants have similar efficacy, and the treatment with estrogen more effectively prevents hot flashes.[42]
Gabapentin reduces spasticity in multiple sclerosis and is prescribed as one of the first-line options.[43] It is an established treatment of restless legs syndrome.[44] Gabapentin alleviates itching in kidney failure (uremic pruritus)[45][46] and itching of other causes.[47] It may be an option in essential or orthostatic tremor.[48][49][50] Although the efficacy of gabapentin for insomnia has not been established, it does alleviate sleep disorder in patients with medical illness.[51]
Gabapentin does not appear to provide benefit for bipolar disorder,[33] complex regional pain syndrome,[52] post-surgical pain,[53] or tinnitus,[54] or prevent episodic migraine in adults.[55]
Contraindications[]
Gabapentin should be used carefully and at lower doses in people with kidney problems due to possible accumulation and toxicity. It is unclear if it is safe during pregnancy or breastfeeding.[5]
Side effects[]
Dizziness and somnolence are the most frequent side effects. Fatigue, ataxia, peripheral edema (swelling of extremities), and nystagmus are also common.[56] Gabapentin is associated with a weight gain of 2.2 kg after 1.5 months of use.[57] Case studies indicate that it may cause anorgasmia and erectile dysfunction,[58] as well as myoclonus[59][60] that disappear after discontinuing gabapentin or replacing it with other medication. DRESS, anaphylaxis, respiratory depression, and increased suicide behavior are the rare but serious side effects.
Suicide[]
The gabapentin label contains a warning of an increased risk of suicidal thoughts and behaviors.[56] According to an insurance claims database study, gabapentin use is associated with about 40% increased risk of suicide, suicide attempt and violent death as compared with a reference anticonvulsant drug topiramate. The risk is increased for both bipolar disorder and epilepsy patients.[61] Another study has shown an approximately doubled rate of suicide attempts and self-harm in patients with bipolar disorder who are taking gabapentin versus those taking lithium.[62]
Respiratory depression[]
Serious breathing suppression, potentially fatal, may occur when gabapentin is taken together with opioids, benzodiazepines, or other depressants, or by people with underlying lung problems such as COPD.[63][64] Gabapentin and opioids are commonly prescribed or abused together, and research indicates that the breathing suppression they cause is additive. For example, gabapentin use before joint replacement or laparoscopic surgery increased the risk of respiratory depression by 30-60%.[63]
Withdrawal and dependence[]
Withdrawal symptoms typically occur 1–2 days after abruptly stopping gabapentin.[65] Agitation, confusion and disorientation are the most frequently reported, followed by gastrointestinal complaints and sweating, and more rare tremor, tachycardia, hypertension, and insomnia.[65] In some cases, users experience withdrawal seizures.[66] All these symptoms subside when gabapentin is re-instated.[65]
On its own, gabapentin appears to not have a substantial addictive power. In human and animal experiments, it shows limited to no rewarding effects. The vast majority of people abusing gabapentin are current or former abusers of opioids or sedatives.[66] In these persons, gabapentin can boost the opioid "high" as well as decrease commonly experienced opioid-withdrawal symptoms such as anxiety.[67]
Overdose[]
Through excessive ingestion, accidental or otherwise, persons may experience overdose symptoms including drowsiness, sedation, blurred vision, slurred speech, somnolence, uncontrollable jerking motions, and anxiety. A very high amount taken is associated with breathing suppression, coma, and possibly death, particularly if combined with alcohol or opioids.[66][68]
Pharmacology[]
Pharmacodynamics[]
Gabapentin is a ligand of the α2δ calcium channel subunit.[69][70] α2δ is an auxiliary protein connected to the main α1 subunit (the channel-forming protein) of high voltage activated voltage-dependent calcium channels (L-type, N-type, P/Q type, and R-type).[9] Gabapentin is not a direct channel blocker: it exerts its actions by disrupting the regulatory function of α2δ and its interactions with other proteins. Gabapentin prevents delivery of the calcium channels to the cell membrane, reduces the activation of the channels by the α2δ subunit, decreases signaling leading to neurotransmitters release, and disrupts interactions of α2δ with NMDA receptors, neurexins, and thrombospondins.[9][10][11] Out of the four known isoforms of α2δ protein, gabapentin binds with similar high affinity to two: α2δ-1 and α2δ-2.[70] Most of the pharmacological properties of gabapentin are explained by its binding to just one isoform - α2δ-1.[70][10]
The endogenous α-amino acids L-leucine and L-isoleucine, which resemble gabapentin in chemical structure, bind α2δ with similar affinity to gabapentin and are present in human cerebrospinal fluid at micromolar concentrations.[71] They may be the endogenous ligands of the α2δ subunit, and they competitively antagonize the effects of gabapentin.[71][72] Accordingly, while gabapentin has nanomolar affinity for the α2δ subunit, its potency in vivo is in the low micromolar range, and competition for binding by endogenous L-amino acids is likely to be responsible for this discrepancy.[10]
Gabapentin is a potent activator of voltage-gated potassium channels KCNQ3 and KCNQ5, even at low nanomolar concentrations. However, this activation is unlikely to be the dominant mechanism of gabapentin's therapeutic effects.[73]
Despite the fact that gabapentin is a structural GABA analogue, and in spite of its name, it does not bind to the GABA receptors, does not convert into GABA or another GABA receptor agonist in vivo, and does not modulate GABA transport or metabolism.[69]
Pharmacokinetics[]
Gabapentin is absorbed from the intestines by an active transport process mediated via an amino acid transporter, presumably, LAT2.[74] As a result, the pharmacokinetics of gabapentin is dose-dependent, with diminished bioavailability and delayed peak levels at higher doses.[70]
The oral bioavailability of gabapentin is approximately 80% at 100 mg administered three times daily once every 8 hours, but decreases to 60% at 300 mg, 47% at 400 mg, 34% at 800 mg, 33% at 1,200 mg, and 27% at 1,600 mg, all with the same dosing schedule.[5][75] Drugs that increase the transit time of gabapentin in the small intestine can increase its oral bioavailability; when gabapentin was co-administered with oral morphine, the oral bioavailability of a 600 mg dose of gabapentin increased by 50%.[75]
Gabapentin at a low dose of 100 mg has a Tmax (time to peak levels) of approximately 1.7 hours, while the Tmax increases to 3 to 4 hours at higher doses.[70] Food does not significantly affect the Tmax of gabapentin and increases the Cmax and area-under-curve levels of gabapentin by approximately 10%.[75]
Gabapentin can cross the blood–brain barrier and enter the central nervous system.[69] Gabapentin concentration in cerebrospinal fluid is approximately 9-14% of its blood plasma concentration.[75] Due to its low lipophilicity,[75] gabapentin requires active transport across the blood–brain barrier.[76][69][77][78] The LAT1 is highly expressed at the blood–brain barrier[79] and transports gabapentin across into the brain.[76][69][77][78] As with intestinal absorption mediated by an amino acid transporter, the transport of gabapentin across the blood–brain barrier by LAT1 is saturable.[76] Gabapentin does not bind to other drug transporters such as P-glycoprotein (ABCB1) or OCTN2 (SLC22A5).[76] It is not significantly bound to plasma proteins (<1%).[75]
Gabapentin undergoes little or no metabolism.[70][75]
Gabapentin is eliminated renally in the urine.[75] It has a relatively short elimination half-life, with the reported average value of 5 to 7 hours.[75] This value changes with increasing doses, from 5.4 hours for a 200 mg single dose, to 8.3 hours for a 1,400 mg dose.[80] Because of its short elimination half-life, gabapentin must be administered 3 to 4 times per day to maintain therapeutic levels.[81] Gabapentin XR (brand name Gralise) is taken once a day.[82]
Chemistry[]

Gabapentin is a 3,3-disubstituted derivative of GABA. Therefore, it is a GABA analogue, as well as a γ-amino acid.[83][84] Specifically, it is a derivative of GABA with a pentyl disubstitution at 3 position, hence, the name - gabapentin, in such a way as to form a six-membered ring. After formation of the ring, the amine and carboxylic groups are not in the same relative positions as they are in the GABA:.[85] they are more conformationally constrained.[86]
Synthesis[]
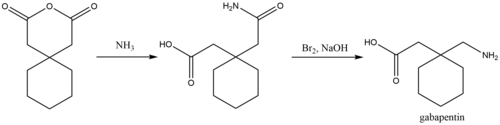
A chemical synthesis of gabapentin has been described starting from 1,1-diacetyl hexane annhydride.[87]
History[]
Gabapentin was designed by researchers at Parke-Davis to be an analogue of the neurotransmitter GABA that could more easily cross the blood–brain barrier and was first described in 1975 by Satzinger and Hartenstein.[85][88] Under the brand name Neurontin, it was first approved in May 1993, for the treatment of epilepsy in the United Kingdom.[89] Approval by the U.S. Food and Drug Administration followed in December 1993, for use as an adjuvant (effective when added to other antiseizure drugs) medication to control partial seizures in adults; that indication was extended to children in 2000.[90][56] Subsequently, gabapentin was approved in the United States for the treatment of postherpetic neuralgia in 2002.[91] A generic version of gabapentin first became available in the United States in 2004.[13] An extended-release formulation of gabapentin for once-daily administration, under the brand name Gralise, was approved in the United States for the treatment postherpetic neuralgia in January 2011.[92][93]
Society and culture[]
Legal status[]
United Kingdom[]
Effective April 2019, the United Kingdom reclassified the drug as a class C controlled substance.[94][95][96][97][98]
United States[]
Effective 1 July 2017, Kentucky classified gabapentin as a schedule V controlled substance statewide.[99] Effective 9 January 2019, Michigan also classified gabapentin as a schedule V controlled substance.[100] Gabapentin is scheduled V drug in other states such as West Virginia,[101] Tennessee,[102] Alabama[103]
Off-label promotion[]
Although some small, non-controlled studies in the 1990s—mostly sponsored by gabapentin's manufacturer—suggested that treatment for bipolar disorder with gabapentin may be promising,[104] the preponderance of evidence suggests that it is not effective.[105] Subsequent to the corporate acquisition of the original patent holder, the pharmaceutical company Pfizer admitted that there had been violations of FDA guidelines regarding the promotion of unproven off-label uses for gabapentin in the Franklin v. Parke-Davis case (see below).
Reuters reported on 25 March 2010, that "Pfizer Inc violated federal racketeering law by improperly promoting the epilepsy drug Neurontin ... Under federal RICO law the penalty is automatically tripled, so the finding will cost Pfizer $141 million."[106] The case stems from a claim from Kaiser Foundation Health Plan Inc. that "it was misled into believing Neurontin was effective for off-label treatment of migraines, bipolar disorder and other conditions. Pfizer argued that Kaiser physicians still recommend the drug for those uses",[107] and that "the insurer's website also still lists Neurontin as a drug for neuropathic pain."[108]
The Wall Street Journal noted that Pfizer spokesman Christopher Loder said, "We are disappointed with the verdict and will pursue post-trial motions and an appeal."[109] He later added that "the verdict and the judge's rulings are not consistent with the facts and the law."[106]
Franklin v. Parke-Davis case[]
While off-label prescriptions are common for a number of drugs, marketing of off-label uses of a drug is not.[16] In 2004, Warner-Lambert (which subsequently was acquired by Pfizer) agreed to plead guilty for activities of its Parke-Davis subsidiary, and to pay $430 million in fines to settle civil and criminal charges regarding the marketing of Neurontin for off-label purposes. The 2004 settlement was one of the largest in U.S. history, and the first off-label promotion case brought successfully under the False Claims Act.[110]
Brand names[]
Gabapentin was originally marketed under the brand name Neurontin. Since it became generic, it has been marketed worldwide using over 300 different brand names.[1] An extended-release formulation of gabapentin for once-daily administration was introduced in 2011 for postherpetic neuralgia under the brand name Gralise.[111]
Related drugs[]
Parke-Davis developed a drug called pregabalin, which is related in structure to gabapentin, as a successor to gabapentin.[112] Another similar drug atagabalin has been unsuccessfully tried by Pfizer as a treatment for insomnia.[113] A prodrug form (gabapentin enacarbil)[114] was approved by the U.S. Food and Drug Administration (FDA).
Recreational use[]
Gabapentin when taken in excess can induce euphoria, a sense of calm, a marijuana-like high, improved sociability, and reduced alcohol or cocaine cravings.[115][116][117] Also known on the streets as "Gabbies",[118] gabapentin is increasingly being abused and misused for these euphoric effects.[119][120] Withdrawal symptoms, often resembling those of benzodiazepine withdrawal, play a role in the physical dependence some users experience.[66] About 1 percent of the responders to an internet poll and 22 percent of those attending addiction facilities had a history of abuse of gabapentin.[65][121] Its misuse predominantly coincides with the usage of other illicit CNS depressant drugs, namely opioids, benzodiazepines, and alcohol.[122] After Kentucky's implementation of stricter legislation regarding opioid prescriptions in 2012, there was an increase in gabapentin-only and multi-drug use in 2012–2015. The majority of these cases were from overdose in suspected suicide attempts. These rates were also accompanied by increases in abuse and recreational use.[123] Gabapentin misuse, toxicity, and use in suicide attempts among adults in the US increased from 2013 to 2017.[124]
Veterinary use[]
In cats, gabapentin can be used as an analgesic in multi-modal pain management,[125] anxiety medication to reduce stress in cats for travel or vet visits,[126] and anticonvulsant.[127] Veterinarians may prescribe gabapentin as an anticonvulsant and pain reliever in dogs. It is also used to treat chronic pain-associated nerve inflammation in horses and dogs. Side effects include tiredness and loss of coordination.[127]
References[]
- ^ Jump up to: a b Drugs.com international listings for Gabapentin Archived 16 February 2016 at the Wayback Machine Page accessed 9 February 2016
- ^ Jump up to: a b "Gabapentin Use During Pregnancy". Drugs.com. 2 December 2019. Retrieved 21 December 2019.
- ^ Jump up to: a b c d e "Neurontin, Gralise (gabapentin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 16 December 2014. Retrieved 6 April 2014.
- ^ Jump up to: a b c d e Goa KL, Sorkin EM (September 1993). "Gabapentin. A review of its pharmacological properties and clinical potential in epilepsy". Drugs. 46 (3): 409–427. doi:10.2165/00003495-199346030-00007. PMID 7693432.
- ^ Jump up to: a b c d e f g "DailyMed - NEURONTIN- gabapentin capsule NEURONTIN- gabapentin tablet, film coated NEURONTIN- gabapentin solution". Retrieved 14 December 2020.
- ^ Jump up to: a b c "1 Recommendations | Neuropathic pain in adults: pharmacological management in non-specialist settings | Guidance | NICE". Retrieved 14 December 2020.
- ^ Jump up to: a b c d e Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, Nurmikko T (September 2010). "EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision". European Journal of Neurology. 17 (9): 1113–e88. doi:10.1111/j.1468-1331.2010.02999.x. PMID 20402746. S2CID 14236933.
- ^ Jump up to: a b c Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, Moore RA (June 2017). "Gabapentin for chronic neuropathic pain in adults". The Cochrane Database of Systematic Reviews. 6 (2): CD007938. doi:10.1002/14651858.CD007938.pub4. hdl:10044/1/52908. PMC 6452908. PMID 28597471.
- ^ Jump up to: a b c Risher WC, Eroglu C (August 2020). "Emerging roles for α2δ subunits in calcium channel function and synaptic connectivity". Curr Opin Neurobiol. 63: 162–169. doi:10.1016/j.conb.2020.04.007. PMC 7483897. PMID 32521436.
- ^ Jump up to: a b c d Stahl SM, Porreca F, Taylor CP, Cheung R, Thorpe AJ, Clair A (2013). "The diverse therapeutic actions of pregabalin: is a single mechanism responsible for several pharmacological activities?". Trends Pharmacol. Sci. 34 (6): 332–9. doi:10.1016/j.tips.2013.04.001. PMID 23642658.
- ^ Jump up to: a b Taylor CP, Harris EW (July 2020). "Analgesia with Gabapentin and Pregabalin May Involve N-Methyl-d-Aspartate Receptors, Neurexins, and Thrombospondins". J Pharmacol Exp Ther. 374 (1): 161–174. doi:10.1124/jpet.120.266056. PMID 32321743. S2CID 216082872.
- ^ Pitkänen A, Schwartzkroin PA, Moshé SL (2005). Models of Seizures and Epilepsy. Burlington: Elsevier. p. 539. ISBN 978-0-08-045702-4. Archived from the original on 8 September 2017.
- ^ Jump up to: a b Reed D (2 March 2012). The Other End of the Stethoscope: The Physician's Perspective on the Health Care Crisis. AuthorHouse. pp. 63–. ISBN 978-1-4685-4410-7.
- ^ "The Top 300 of 2021". ClinCalc. Retrieved 18 February 2021.
- ^ "Gabapentin - Drug Usage Statistics". ClinCalc. Retrieved 18 February 2021.
- ^ Jump up to: a b Henney JE (August 2006). "Safeguarding patient welfare: who's in charge?". Annals of Internal Medicine. 145 (4): 305–7. doi:10.7326/0003-4819-145-4-200608150-00013. PMID 16908923. S2CID 39262014.
- ^ Stempel J (2 June 2014). "Pfizer to pay $325 million in Neurontin settlement". Reuters. Retrieved 11 June 2018.
- ^ Jump up to: a b Montastruc F, Loo SY, Renoux C (November 2018). "Trends in First Gabapentin and Pregabalin Prescriptions in Primary Care in the United Kingdom, 1993-2017". JAMA. 320 (20): 2149–2151. doi:10.1001/jama.2018.12358. PMC 6583557. PMID 30480717.
- ^ Goodman CW, Brett AS (May 2019). "A Clinical Overview of Off-label Use of Gabapentinoid Drugs". JAMA Intern Med. 179 (5): 695–701. doi:10.1001/jamainternmed.2019.0086. PMID 30907944.
- ^ Jump up to: a b Sobel SV (5 November 2012). Successful Psychopharmacology: Evidence-Based Treatment Solutions for Achieving Remission. W. W. Norton. p. 124. ISBN 978-0-393-70857-8. Archived from the original on 6 January 2016.
- ^ Johannessen SI, Ben-Menachem E (2006). "Management of focal-onset seizures: an update on drug treatment". Drugs. 66 (13): 1701–25. doi:10.2165/00003495-200666130-00004. PMID 16978035. S2CID 46952737.
- ^ Rheims S, Ryvlin P (July 2014). "Pharmacotherapy for tonic-clonic seizures". Expert Opin Pharmacother. 15 (10): 1417–26. doi:10.1517/14656566.2014.915029. PMID 24798217. S2CID 6943460.
- ^ Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, Coderre T, Morley-Forster PK, Stinson J, Boulanger A, Peng P, Finley GA, Taenzer P, Squire P, Dion D, Cholkan A, Gilani A, Gordon A, Henry J, Jovey R, Lynch M, Mailis-Gagnon A, Panju A, Rollman GB, Velly A (2007). "Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society". Pain Res Manag. 12 (1): 13–21. doi:10.1155/2007/730785. PMC 2670721. PMID 17372630.
- ^ Jump up to: a b c Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M (February 2015). "Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis". Lancet Neurol. 14 (2): 162–73. doi:10.1016/S1474-4422(14)70251-0. PMC 4493167. PMID 25575710.
- ^ Shanthanna H, Gilron I, Rajarathinam M, AlAmri R, Kamath S, Thabane L, Devereaux PJ, Bhandari M (August 2017). "Benefits and safety of gabapentinoids in chronic low back pain: A systematic review and meta-analysis of randomized controlled trials". PLOS Medicine. 14 (8): e1002369. doi:10.1371/journal.pmed.1002369. PMC 5557428. PMID 28809936.
- ^ Enke O, New HA, New CH, Mathieson S, McLachlan AJ, Latimer J, Maher CG, Lin CC (July 2018). "Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis". CMAJ. 190 (26): E786–E793. doi:10.1503/cmaj.171333. PMC 6028270. PMID 29970367.
- ^ Phillips TJ, Cherry CL, Cox S, Marshall SJ, Rice AS (December 2010). Pai (ed.). "Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials". PLOS ONE. 5 (12): e14433. Bibcode:2010PLoSO...514433P. doi:10.1371/journal.pone.0014433. PMC 3010990. PMID 21203440.
- ^ Moore A, Derry S, Wiffen P (February 2018). "Gabapentin for Chronic Neuropathic Pain". JAMA. 319 (8): 818–819. doi:10.1001/jama.2017.21547. PMID 29486015.
- ^ Jump up to: a b c Mula M, Pini S, Cassano GB (June 2007). "The role of anticonvulsant drugs in anxiety disorders: a critical review of the evidence". Journal of Clinical Psychopharmacology. 27 (3): 263–72. doi:10.1097/jcp.0b013e318059361a. PMID 17502773. S2CID 38188832.
- ^ Jump up to: a b c Greenblatt HK, Greenblatt DJ (March 2018). "Gabapentin and Pregabalin for the Treatment of Anxiety Disorders". Clin Pharmacol Drug Dev. 7 (3): 228–232. doi:10.1002/cpdd.446. PMID 29579375. S2CID 4321472.
- ^ Schatzberg A (2015). Manual of clinical psychopharmacology. Washington, DC: American Psychiatric Publishing, a division of American Psychiatric Association. p. 374. ISBN 978-1-58562-481-2. OCLC 902727278.
- ^ Muncie HL, Yasinian Y, Oge' L (November 2013). "Outpatient management of alcohol withdrawal syndrome". American Family Physician. 88 (9): 589–95. PMID 24364635.
- ^ Jump up to: a b Berlin RK, Butler PM, Perloff MD (2015). "Gabapentin Therapy in Psychiatric Disorders: A Systematic Review". The Primary Care Companion for CNS Disorders. 17 (5). doi:10.4088/PCC.15r01821. PMC 4732322. PMID 26835178.
- ^ Ahmed S, Stanciu CN, Kotapati PV, Ahmed R, Bhivandkar S, Khan AM, Afridi A, Qureshi M, Esang M (August 2019). "Effectiveness of Gabapentin in Reducing Cravings and Withdrawal in Alcohol Use Disorder: A Meta-Analytic Review". Prim Care Companion CNS Disord. 21 (4). doi:10.4088/PCC.19r02465. PMID 31461226.
- ^ Kranzler HR, Feinn R, Morris P, Hartwell EE (September 2019). "A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder". Addiction. 114 (9): 1547–1555. doi:10.1111/add.14655. PMC 6682454. PMID 31077485.
- ^ Jump up to: a b Mason BJ, Quello S, Shadan F (January 2018). "Gabapentin for the treatment of alcohol use disorder". Expert Opin Investig Drugs. 27 (1): 113–124. doi:10.1080/13543784.2018.1417383. PMC 5957503. PMID 29241365.
- ^ Sood A, Ebbert JO, Wyatt KD, Croghan IT, Schroeder DR, Sood R, Hays JT (March 2010). "Gabapentin for smoking cessation". Nicotine & Tobacco Research. 12 (3): 300–4. doi:10.1093/ntr/ntp195. PMC 2825098. PMID 20081039.
- ^ Nielsen S, Gowing L, Sabioni P, Le Foll B (January 2019). "Pharmacotherapies for cannabis dependence". The Cochrane Database of Systematic Reviews. 1 (3): CD008940. doi:10.1002/14651858.CD008940.pub3. PMC 6360924. PMID 30687936.
- ^ McLean RJ, Gottlob I (August 2009). "The pharmacological treatment of nystagmus: a review". Expert Opinion on Pharmacotherapy. 10 (11): 1805–16. doi:10.1517/14656560902978446. PMID 19601699. S2CID 21477128.
- ^ Thurtell MJ, Leigh RJ (February 2012). "Treatment of nystagmus". Curr Treat Options Neurol. 14 (1): 60��72. doi:10.1007/s11940-011-0154-5. PMID 22072056. S2CID 40370476.
- ^ Mehta AR, Kennard C (June 2012). "The pharmacological treatment of acquired nystagmus". Pract Neurol. 12 (3): 147–53. doi:10.1136/practneurol-2011-000181. PMID 22661344. S2CID 1950738.
- ^ Shan D, Zou L, Liu X, Shen Y, Cai Y, Zhang J (June 2020). "Efficacy and safety of gabapentin and pregabalin in patients with vasomotor symptoms: a systematic review and meta-analysis". Am J Obstet Gynecol. 222 (6): 564–579.e12. doi:10.1016/j.ajog.2019.12.011. PMID 31870736.
- ^ Otero-Romero S, Sastre-Garriga J, Comi G, Hartung HP, Soelberg Sørensen P, Thompson AJ, Vermersch P, Gold R, Montalban X (October 2016). "Pharmacological management of spasticity in multiple sclerosis: Systematic review and consensus paper". Mult Scler. 22 (11): 1386–1396. doi:10.1177/1352458516643600. PMID 27207462. S2CID 25028259.
- ^ Winkelmann J, Allen RP, Högl B, Inoue Y, Oertel W, Salminen AV, Winkelman JW, Trenkwalder C, Sampaio C (July 2018). "Treatment of restless legs syndrome: Evidence-based review and implications for clinical practice (Revised 2017)§". Mov Disord. 33 (7): 1077–1091. doi:10.1002/mds.27260. PMID 29756335. S2CID 21669996.
- ^ Berger TG, Steinhoff M (June 2011). "Pruritus and renal failure". Seminars in Cutaneous Medicine and Surgery. 30 (2): 99–100. doi:10.1016/j.sder.2011.04.005. PMC 3692272. PMID 21767770.
- ^ Hercz D, Jiang SH, Webster AC (December 2020). "Interventions for itch in people with advanced chronic kidney disease". Cochrane Database Syst Rev. 12: CD011393. doi:10.1002/14651858.CD011393.pub2. PMC 8094883. PMID 33283264.
- ^ Anand S (March 2013). "Gabapentin for pruritus in palliative care". The American Journal of Hospice & Palliative Care. 30 (2): 192–6. doi:10.1177/1049909112445464. PMID 22556282. S2CID 39737885.
- ^ Schneider SA, Deuschl G (January 2014). "The treatment of tremor". Neurotherapeutics. 11 (1): 128–38. doi:10.1007/s13311-013-0230-5. PMC 3899476. PMID 24142589.
- ^ Zesiewicz TA, Elble RJ, Louis ED, Gronseth GS, Ondo WG, Dewey RB, Okun MS, Sullivan KL, Weiner WJ (November 2011). "Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology". Neurology. 77 (19): 1752–5. doi:10.1212/WNL.0b013e318236f0fd. PMC 3208950. PMID 22013182.
- ^ Sadeghi R, Ondo WG (December 2010). "Pharmacological management of essential tremor". Drugs. 70 (17): 2215–28. doi:10.2165/11538180-000000000-00000. PMID 21080739. S2CID 10662268.
- ^ Liu GJ, Karim MR, Xu LL, Wang SL, Yang C, Ding L, Wang YF (2017). "Efficacy and Tolerability of Gabapentin in Adults with Sleep Disturbance in Medical Illness: A Systematic Review and Meta-analysis". Front Neurol. 8: 316. doi:10.3389/fneur.2017.00316. PMC 5510619. PMID 28769860.
- ^ Tran DQ, Duong S, Bertini P, Finlayson RJ (February 2010). "Treatment of complex regional pain syndrome: a review of the evidence". Canadian Journal of Anaesthesia. 57 (2): 149��66. doi:10.1007/s12630-009-9237-0. PMID 20054678.
- ^ Hamilton TW, Strickland LH, Pandit HG (August 2016). "A Meta-Analysis on the Use of Gabapentinoids for the Treatment of Acute Postoperative Pain Following Total Knee Arthroplasty". The Journal of Bone and Joint Surgery. American Volume. 98 (16): 1340–50. doi:10.2106/jbjs.15.01202. PMID 27535436.
- ^ Aazh H, El Refaie A, Humphriss R (December 2011). "Gabapentin for tinnitus: a systematic review". American Journal of Audiology. 20 (2): 151–8. doi:10.1044/1059-0889(2011/10-0041). PMID 21940981.
- ^ Linde M, Mulleners WM, Chronicle EP, McCrory DC (June 2013). "Gabapentin or pregabalin for the prophylaxis of episodic migraine in adults". The Cochrane Database of Systematic Reviews. 6 (6): CD010609. doi:10.1002/14651858.CD010609. PMC 6599858. PMID 23797675.
- ^ Jump up to: a b c "Neurontin- gabapentin capsule Neurontin- gabapentin tablet, film coated Neurontin- gabapentin solution". DailyMed. 11 April 2019. Retrieved 21 December 2019.
- ^ Domecq JP, Prutsky G, Leppin A, Sonbol MB, Altayar O, Undavalli C, Wang Z, Elraiyah T, Brito JP, Mauck KF, Lababidi MH, Prokop LJ, Asi N, Wei J, Fidahussein S, Montori VM, Murad MH (February 2015). "Clinical review: Drugs commonly associated with weight change: a systematic review and meta-analysis". J Clin Endocrinol Metab. 100 (2): 363–70. doi:10.1210/jc.2014-3421. PMC 5393509. PMID 25590213.
- ^ Yang Y, Wang X (January 2016). "Sexual dysfunction related to antiepileptic drugs in patients with epilepsy". Expert Opin Drug Saf. 15 (1): 31–42. doi:10.1517/14740338.2016.1112376. PMID 26559937. S2CID 39571068.
- ^ Kim JB, Jung JM, Park MH, Lee EJ, Kwon DY (November 2017). "Negative myoclonus induced by gabapentin and pregabalin: A case series and systematic literature review". J Neurol Sci. 382: 36–39. doi:10.1016/j.jns.2017.09.019. PMID 29111014. S2CID 32010921.
- ^ Desai A, Kherallah Y, Szabo C, Marawar R (March 2019). "Gabapentin or pregabalin induced myoclonus: A case series and literature review". J Clin Neurosci. 61: 225–234. doi:10.1016/j.jocn.2018.09.019. PMID 30381161. S2CID 53165515.
- ^ Patorno E, Bohn RL, Wahl PM, Avorn J, Patrick AR, Liu J, Schneeweiss S (April 2010). "Anticonvulsant medications and the risk of suicide, attempted suicide, or violent death". JAMA. 303 (14): 1401–9. doi:10.1001/jama.2010.410. PMID 20388896.
- ^ Leith WM, Lambert WE, Boehnlein JK, Freeman MD (January 2019). "The association between gabapentin and suicidality in bipolar patients". International Clinical Psychopharmacology. 34 (1): 27–32. doi:10.1097/YIC.0000000000000242. PMID 30383553. S2CID 54130760.
- ^ Jump up to: a b "FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR)". U.S. Food and Drug Administration (FDA). 19 December 2019. Archived from the original on 22 December 2019. Retrieved 21 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR) When used with CNS depressants or in patients with lung problems" (PDF). 19 December 2019. Archived from the original on 22 December 2019. Retrieved 22 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Jump up to: a b c d Mersfelder TL, Nichols WH (March 2016). "Gabapentin: Abuse, Dependence, and Withdrawal". The Annals of Pharmacotherapy. 50 (3): 229–33. doi:10.1177/1060028015620800. PMID 26721643. S2CID 21108959.
- ^ Jump up to: a b c d Bonnet U, Scherbaum N (December 2017). "How addictive are gabapentin and pregabalin? A systematic review". European Neuropsychopharmacology. 27 (12): 1185–1215. doi:10.1016/j.euroneuro.2017.08.430. PMID 28988943. S2CID 10345555.
- ^ Bonnet U, Richter EL, Isbruch K, Scherbaum N (June 2018). "On the addictive power of gabapentinoids: a mini-review" (PDF). Psychiatria Danubina. 30 (2): 142–149. doi:10.24869/psyd.2018.142. PMID 29930223.
- ^ R.C. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 677–8. ISBN 978-0-9626523-7-0.
- ^ Jump up to: a b c d e Sills GJ (February 2006). "The mechanisms of action of gabapentin and pregabalin". Current Opinion in Pharmacology. 6 (1): 108–13. doi:10.1016/j.coph.2005.11.003. PMID 16376147.
- ^ Jump up to: a b c d e f Calandre EP, Rico-Villademoros F, Slim M (November 2016). "Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use". Expert Rev Neurother. 16 (11): 1263–1277. doi:10.1080/14737175.2016.1202764. PMID 27345098. S2CID 33200190.
- ^ Jump up to: a b Dooley DJ, Taylor CP, Donevan S, Feltner D (February 2007). "Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission". Trends in Pharmacological Sciences. 28 (2): 75–82. doi:10.1016/j.tips.2006.12.006. PMID 17222465.
- ^ Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC (May 2007). "Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels". Trends in Pharmacological Sciences. 28 (5): 220–8. doi:10.1016/j.tips.2007.03.005. PMID 17403543.
- ^ Manville RW, Abbott GW (October 2018). "Gabapentin Is a Potent Activator of KCNQ3 and KCNQ5 Potassium Channels". Mol Pharmacol. 94 (4): 1155–1163. doi:10.1124/mol.118.112953. PMC 6108572. PMID 30021858.
- ^ del Amo EM, Urtti A, Yliperttula M (October 2008). "Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2". Eur J Pharm Sci. 35 (3): 161–74. doi:10.1016/j.ejps.2008.06.015. PMID 18656534.
- ^ Jump up to: a b c d e f g h i Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P (October 2010). "A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin". Clinical Pharmacokinetics. 49 (10): 661–9. doi:10.2165/11536200-000000000-00000. PMID 20818832. S2CID 16398062.
- ^ Jump up to: a b c d Dickens D, Webb SD, Antonyuk S, Giannoudis A, Owen A, Rädisch S, Hasnain SS, Pirmohamed M (June 2013). "Transport of gabapentin by LAT1 (SLC7A5)". Biochemical Pharmacology. 85 (11): 1672–83. doi:10.1016/j.bcp.2013.03.022. PMID 23567998.
- ^ Jump up to: a b Geldenhuys WJ, Mohammad AS, Adkins CE, Lockman PR (2015). "Molecular determinants of blood-brain barrier permeation". Therapeutic Delivery. 6 (8): 961–71. doi:10.4155/tde.15.32. PMC 4675962. PMID 26305616.
- ^ Jump up to: a b Müller CE (November 2009). "Prodrug approaches for enhancing the bioavailability of drugs with low solubility". Chemistry & Biodiversity. 6 (11): 2071–83. doi:10.1002/cbdv.200900114. PMID 19937841. S2CID 32513471.
- ^ Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM (October 1999). "Selective expression of the large neutral amino acid transporter at the blood-brain barrier". Proceedings of the National Academy of Sciences of the United States of America. 96 (21): 12079–84. Bibcode:1999PNAS...9612079B. doi:10.1073/pnas.96.21.12079. PMC 18415. PMID 10518579.
- ^ Cundy KC, Sastry S, Luo W, Zou J, Moors TL, Canafax DM (December 2008). "Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin". Journal of Clinical Pharmacology. 48 (12): 1378–88. doi:10.1177/0091270008322909. PMID 18827074. S2CID 23598218.
- ^ Agarwal P, Griffith A, Costantino HR, Vaish N (May 2010). "Gabapentin enacarbil - clinical efficacy in restless legs syndrome". Neuropsychiatric Disease and Treatment. 6: 151–8. doi:10.2147/NDT.S5712. PMC 2874339. PMID 20505847.
- ^ Kaye AD (5 June 2017). Pharmacology, An Issue of Anesthesiology Clinics E-Book. Elsevier Health Sciences. pp. 98–. ISBN 978-0-323-52998-3.
- ^ Wyllie E, Cascino GD, Gidal BE, Goodkin HP (17 February 2012). Wyllie's Treatment of Epilepsy: Principles and Practice. Lippincott Williams & Wilkins. p. 423. ISBN 978-1-4511-5348-4.
- ^ Benzon H, Rathmell JP, Wu CL, Turk DC, Argoff CE, Hurley RW (11 September 2013). Practical Management of Pain. Elsevier Health Sciences. p. 1006. ISBN 978-0-323-17080-2.
- ^ Jump up to: a b Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. pp. 219–220. ISBN 978-0-470-01552-0.
- ^ Levandovskiy IA, Sharapa DI, Shamota TV, Rodionov VN, Shubina TE (February 2011). "Conformationally restricted GABA analogs: from rigid carbocycles to cage hydrocarbons". Future Medicinal Chemistry. 3 (2): 223–41. doi:10.4155/fmc.10.287. PMID 21428817.
- ^ "pdfs.semanticscholar.org" (PDF). US Patent Application US 2008/0103334 A1. S2CID 55768537. Retrieved 21 December 2020.
- ^ Johnson DS, Li JJ (26 February 2013). The Art of Drug Synthesis. John Wiley & Sons. pp. 13–. ISBN 978-1-118-67846-6.
- ^ "Drug Profile: Gabapentin". Adis Insight.
- ^ Mack A (2003). "Examination of the evidence for off-label use of gabapentin" (PDF). Journal of Managed Care Pharmacy. 9 (6): 559–68. doi:10.18553/jmcp.2003.9.6.559. PMID 14664664. Archived from the original (PDF) on 17 September 2010. Retrieved 15 August 2006.
- ^ Irving G (September 2012). "Once-daily gastroretentive gabapentin for the management of postherpetic neuralgia: an update for clinicians". Therapeutic Advances in Chronic Disease. 3 (5): 211–8. doi:10.1177/2040622312452905. PMC 3539268. PMID 23342236.
- ^ Orrange S (31 May 2013). "Yabba Dabba Gabapentin: Are Gralise and Horizant Worth the Cost?". GoodRx, Inc. Archived from the original on 23 June 2018. Retrieved 22 June 2018.
- ^ "Gabapentin controlled release - Depomed". Adis Insight.
- ^ "Pregabalin and gabapentin will become controlled drugs in April". NursingNotes. 17 October 2018. Retrieved 16 June 2019.
- ^ "Re: Pregabalin and Gabapentin advice" (PDF). GOV.UK. 14 January 2016.
- ^ "Pregabalin and gabapentin: proposal to schedule under the Misuse of Drugs Regulations 2001". GOV.UK. 10 November 2017. Retrieved 2 April 2020.
- ^ Mayor S (16 October 2018). "Pregabalin and gabapentin become controlled drugs to cut deaths from misuse". British Medical Journal. 363: k4364. doi:10.1136/bmj.k4364. ISSN 0959-8138. PMID 30327316. S2CID 53520780.
- ^ "Pregabalin and gabapentin to be controlled as Class C drugs". GOV.UK. 15 October 2018. Retrieved 2 April 2020.
- ^ "Important Notice: Gabapentin Becomes a Schedule 5 Controlled Substance in Kentucky" (PDF). Kentucky State Board of Pharmacy. March 2017. Retrieved 18 June 2018.
- ^ "Gabapentin Scheduled as Controlled Substance to help with State's Opioid Epidemic". Retrieved 16 April 2019.
- ^ "WV Code 212". www.wvlegislature.gov. Retrieved 30 May 2020.
- ^ "Gabapentin will be a Schedule V controlled substance in Tennessee effective July 1, 2018" (PDF). tn.gov. Retrieved 30 May 2020.
- ^ "Pharmacy Division | Alabama Department of Public Health (ADPH)". www.alabamapublichealth.gov. Retrieved 30 May 2020.
- ^ Mack A (2003). "Examination of the evidence for off-label use of gabapentin". Journal of Managed Care Pharmacy. 9 (6): 559–568. doi:10.18553/jmcp.2003.9.6.559. PMID 14664664. S2CID 17085492.
- ^ Reinares M, Rosa AR, Franco C, Goikolea JM, Fountoulakis K, Siamouli M, Gonda X, Frangou S, Vieta E (March 2013). "A systematic review on the role of anticonvulsants in the treatment of acute bipolar depression". The International Journal of Neuropsychopharmacology. 16 (2): 485–96. doi:10.1017/S1461145712000491. PMID 22575611.
- ^ Jump up to: a b Berkrot B (25 March 2010). "US jury's Neurontin ruling to cost Pfizer $141 mln". Reuters. Archived from the original on 19 October 2015.
- ^ "Pfizer faces $142M in damages for drug fraud". Bloomberg Businessweek. 25 March 2010. Archived from the original on 19 October 2015. Retrieved 13 January 2012.
- ^ Van Voris B, Lawrence J (26 March 2010). "Pfizer Told to Pay $142.1 Million for Neurontin Marketing Fraud". Bloomberg News. Archived from the original on 13 May 2013. Retrieved 13 January 2012.
- ^ Kamp J (25 March 2010). "Jury Rules Against Pfizer in Marketing Case". The Wall Street Journal. Archived from the original on 19 October 2015. Retrieved 13 January 2012.
- ^ Tansey B (14 May 2004). "Huge penalty in drug fraud, Pfizer settles felony case in Neurontin off-label promotion". San Francisco Chronicle. p. C-1. Archived from the original on 23 June 2006.
- ^ "Gralise Approval History". Drugs.com.
- ^ Baillie JK, Power I (January 2006). "The mechanism of action of gabapentin in neuropathic pain". Current Opinion in Investigational Drugs. 7 (1): 33–9. PMID 16425669.
- ^ Kjellsson MC, Ouellet D, Corrigan B, Karlsson MO (October 2011). "Modeling sleep data for a new drug in development using markov mixed-effects models". Pharmaceutical Research. 28 (10): 2610–27. doi:10.1007/s11095-011-0490-x. PMID 21681607. S2CID 22241527.
- ^ Landmark CJ, Johannessen SI (February 2008). "Modifications of antiepileptic drugs for improved tolerability and efficacy". Perspectives in Medicinal Chemistry. 2: 21–39. doi:10.1177/1177391X0800200001. PMC 2746576. PMID 19787095.
- ^ Smith BH, Higgins C, Baldacchino A, Kidd B, Bannister J (August 2012). "Substance misuse of gabapentin". The British Journal of General Practice : The Journal of the Royal College of General Practitioners. 62 (601): 406–7. doi:10.3399/bjgp12X653516. PMC 3404313. PMID 22867659.
- ^ Shebak S, Varipapa R, Snyder A, Whitham MD, Milam TR (2014). "Gabapentin abuse and overdose: a case report" (PDF). J Subst Abus Alcohol. 2: 1018. S2CID 8959463. Archived from the original (PDF) on 2 March 2019.
- ^ Martinez GM, Olabisi J, Ruekert L, Hasan S (July 2019). "A Call for Caution in Prescribing Gabapentin to Individuals With Concurrent Polysubstance Abuse: A Case Report". Journal of Psychiatric Practice. 25 (4): 308–312. doi:10.1097/PRA.0000000000000403. PMID 31291212.
- ^ Trestman RL, Appelbaum KL, Metzner JL (April 2015). Oxford Textbook of Correctional Psychiatry. Oxford University Press. p. 167. ISBN 978-0199360574.
- ^ Goodman CW, Brett AS (August 2017). "Gabapentin and Pregabalin for Pain – Is Increased Prescribing a Cause for Concern?". The New England Journal of Medicine. 377 (5): 411–414. doi:10.1056/NEJMp1704633. PMID 28767350.
- ^ Evoy KE, Morrison MD, Saklad SR (March 2017). "Abuse and Misuse of Pregabalin and Gabapentin". Drugs. 77 (4): 403–426. doi:10.1007/s40265-017-0700-x. PMID 28144823. S2CID 24396685.
- ^ Bonnet U, Scherbaum N (February 2018). "[On the risk of dependence on gabapentinoids]". Fortschritte der Neurologie-Psychiatrie (in German). 86 (2): 82–105. doi:10.1055/s-0043-122392. PMID 29179227.
- ^ Smith RV, Havens JR, Walsh SL (July 2016). "Gabapentin misuse, abuse and diversion: a systematic review". Addiction. 111 (7): 1160–74. doi:10.1111/add.13324. PMC 5573873. PMID 27265421.
- ^ Faryar KA, Webb AN, Bhandari B, Price TG, Bosse GM (June 2019). "Trending gabapentin exposures in Kentucky after legislation requiring use of the state prescription drug monitoring program for all opioid prescriptions.". Clinical Toxicology. 57 (6): 398–403. doi:10.1080/15563650.2018.1538518. PMID 30676102. S2CID 59226292.
- ^ Reynolds K, Kaufman R, Korenoski A, Fennimore L, Shulman J, Lynch M (July 2020). "Trends in gabapentin and baclofen exposures reported to U.S. poison centers". Clinical Toxicology. 58 (7): 763–772. doi:10.1080/15563650.2019.1687902. PMID 31786961. S2CID 208537638.
- ^ Vettorato E, Corletto F (September 2011). "Gabapentin as part of multi-modal analgesia in two cats suffering multiple injuries". Veterinary Anaesthesia and Analgesia. 38 (5): 518–20. doi:10.1111/j.1467-2995.2011.00638.x. PMID 21831060.
- ^ van Haaften KA, Forsythe LR, Stelow EA, Bain MJ (November 2017). "Effects of a single preappointment dose of gabapentin on signs of stress in cats during transportation and veterinary examination". Journal of the American Veterinary Medical Association. 251 (10): 1175–1181. doi:10.2460/javma.251.10.1175. PMID 29099247.
- ^ Jump up to: a b "Gabapentin". Plumb's Veterinary Drugs. Retrieved 2 April 2021.
External links[]
| Scholia has a topic profile for Gabapentin. |
- "Gabapentin". Drug Information Portal. U.S. National Library of Medicine.
- Amino acids
- Analgesics
- Anticonvulsants
- Anxiolytics
- Calcium channel blockers
- GABA analogues
- Pfizer brands