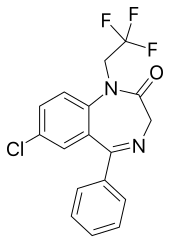
Halazepam Other names 9-chloro-6-phenyl-2-(2,2,2-trifluoroethyl)-2,5-diazabicyclo[5.4.0]undeca-5,8,10,12-tetraen-3-one AHFS /Drugs.com Micromedex Detailed Consumer Information MedlinePlus a684001 Pregnancy Routes of Oral ATC code Legal status
CA Schedule IV DE Anlage III for higher doses) US :Schedule IV
Metabolism Hepatic Elimination half-life 14 hours (halazepam), 50–100 hours (metabolites). Excretion Renal
7-chloro-5-phenyl-1-(2,2,2-trifluoroethyl)-3H -1,4-benzodiazepin-2-one
CAS Number PubChem CID IUPHAR/BPS DrugBank ChemSpider UNII KEGG ChEMBL CompTox Dashboard (EPA ) ECHA InfoCard 100.041.281 Formula C 17 H 12 Cl F 3 N 2 O Molar mass −1 3D model (JSmol )
FC(F)(CN1C(CN=C(C2=CC=CC=C2)C3=C1C=CC(Cl)=C3)=O)F
InChI=1S/C17H12ClF3N2O/c18-12-6-7-14-13(8-12)16(11-4-2-1-3-5-11)22-9-15(24)23(14)10-17(19,20)21/h1-8H,9-10H2
Y Key:WYCLKVQLVUQKNZ-UHFFFAOYSA-N
Y
Halazepam is a benzodiazepine derivative that was marketed under the brand names Paxipam in the United States,[1] Alapryl in Spain,[2] Pacinone in Portugal.[3]
Medical uses [ ] Halazepam was used for the treatment of anxiety .[1]
Adverse effects [ ] Adverse effects include drowsiness, confusion, dizziness, and sedation. Gastrointestinal side effects have also been reported including dry mouth and nausea.[1]
Pharmacokinetics and pharmacodynamics [ ] Pharmacokinetics and pharmacodynamics were listed in Current Psychotherapeutic Drugs published on June 15, 1998 as follows:[4]
Onset of action
Intermediate to slow
Plasma half life
14 hr for parent drug and 30-100 hr for its metabolite
Peak plasma levels
1-3 hr for parent drug and 3-6 hf for its metabolite
Metabolism
Metabolized into desmethyldiazepam and 3-hydroxyhalazepam (in the liver)
Excretion
Excreted through kidneys
Protein binding
98% bound to plasma protein
Regulatory Information [ ] Halazepam is classified as a schedule 4 controlled substance with a corresponding code 2762 by the Drug Enforcement Administration (DEA).[5]
Commercial production [ ] Halazepam was invented by Schlesinger Walter in the U.S. It was marketed as an anti-anxiety agent in 1981. However, Halazepam is not commercially available in the United States because it was withdrawn by its manufacturer for poor sales.[1]
See also [ ] References [ ]
^ a b c d "halazepam" . Drugs.com. Retrieved December 11, 2014 .^ "Alapryl" . Drugs.com. Retrieved December 11, 2014 .^ "Pacinone" . Drugs.com. Retrieved December 11, 2014 .^ Sellers EM (1998). "Antianxiety agents: benzodiazepine derivatives". In Quitkin FM, et al. (eds.). Current Psychotherapeutic Drugs (2nd ed.). Washington: American Psychiatric Press. p. 166. ISBN 978-0-88048-994-2 ^ "SCHEDULES OF CONTROLLED SUBSTANCES" . Code of Federal Regulations. 2012-04-01. pp. § 1308.14 Schedule IV. Retrieved December 12, 2014 .
External links [ ]
Benzodiazepines
1,4-Benzodiazepines 1,5-Benzodiazepines 2,3-Benzodiazepines* Triazolobenzodiazepines Imidazobenzodiazepines
Bretazenil Climazolam EVT-201 FG-8205 Flumazenil GL-II-73 Imidazenil 123 I-IomazenilL-655,708 Loprazolam Midazolam PWZ-029 Remimazolam Ro15-4513 Ro48-6791 Ro48-8684 Ro4938581 Sarmazenil SH-053-R-CH3-2′F Oxazolobenzodiazepines Thienodiazepines Thienotriazolodiazepines Thienobenzodiazepines *Pyridodiazepines Pyridotriazolodiazepines Pyrazolodiazepines Pyrrolodiazepines Tetrahydroisoquinobenzodiazepines Pyrrolobenzodiazepines *Benzodiazepine prodrugs * atypical activity profile (not GABAA receptor ligands)
Anxiolytics (N05B )
5-HT1A R agonists GABAA R PAMs
Benzodiazepines :Adinazolam Alprazolam Bromazepam Camazepam Chlordiazepoxide Clobazam Clonazepam Clorazepate Clotiazepam Cloxazolam Diazepam # Ethyl loflazepate Etizolam Fludiazepam Halazepam Ketazolam Lorazepam # Medazepam Nordazepam Oxazepam Pinazepam Prazepam ; Others: Alpidem ‡ Barbiturates (e.g., phenobarbital )Carisoprodol Carbamates (e.g., meprobamate )Chlormezanone ‡ Ethanol (alcohol) Etifoxine Imepitoin ; Herbs: Kava Skullcap Valerian Gabapentinoids (α2 δ VDCC blockers )
Gabapentin Gabapentin enacarbil Phenibut Pregabalin Antidepressants
SSRIs escitalopram )SNRIs duloxetine )SARIs trazodone )TCAs clomipramine # )TeCAs mirtazapine )MAOIs phenelzine ); Others: Agomelatine Bupropion Tianeptine Vilazodone Vortioxetine Sympatholytics (Antiadrenergics )
Alpha-1 blockers (e.g., prazosin )Alpha-2 agonists (e.g., clonidine , dexmedetomidine , guanfacine )Beta blockers (e.g., propranolol ) Others
Benzoctamine Cannabidiol Cycloserine Fabomotizole Hydroxyzine Kanna Lavender Lorpiprazole Mebicar Mepiprazole Nicotine Opipramol Oxaflozane ‡ Phenaglycodol Phenibut Picamilon Selank Tiagabine Tofisopam Validolum
# WHO-EM ‡ Withdrawn from marketClinical trials :
† Phase III § Never to phase III
GABA A receptor positive modulatorsAlcohols
Butanol Chloralodol Chlorobutanol (cloretone) Ethanol (alcohol) (alcoholic drink )Ethchlorvynol Isobutanol Isopropanol Menthol Methanol Methylpentynol Pentanol Petrichloral Propanol tert -Butanol (2M2P)tert -Pentanol (2M2B)Tribromoethanol Trichloroethanol Triclofos Trifluoroethanol Barbiturates Benzodiazepines Carbamates Flavonoids
Ampelopsin (dihydromyricetin) Apigenin Baicalein Baicalin Catechin EGC EGCG Hispidulin Luteolin Skullcap constituents (e.g., baicalin )Wogonin Imidazoles Kava constituents
Desmethoxyyangonin Kavain Methysticin Yangonin Monoureides Neuroactive steroids Nonbenzodiazepines Phenols
Fospropofol Propofol Thymol Piperidinediones Pyrazolopyridines Quinazolinones Volatiles /gases
Acetone Acetophenone Acetylglycinamide chloral hydrate Aliflurane Benzene Butane Butylene Centalun Chloral Chloral betaine Chloral hydrate Chloroform Cryofluorane Desflurane Dichloralphenazone Dichloromethane Diethyl ether Enflurane Ethyl chloride Ethylene Fluroxene Gasoline Halopropane Halothane Isoflurane Kerosine Methoxyflurane Methoxypropane Nitric oxide Nitrogen Nitrous oxide Norflurane Paraldehyde Propane Propylene Roflurane Sevoflurane Synthane Teflurane Toluene Trichloroethane (methyl chloroform) Trichloroethylene Vinyl ether Others/unsorted
3-Hydroxybutanal Avermectins (e.g., ivermectin )Bromide compounds (e.g., lithium bromide , potassium bromide , sodium bromide )Carbamazepine Chloralose Chlormezanone Clomethiazole DEABL Dihydroergolines (e.g., dihydroergocryptine , , dihydroergotamine , ergoloid (dihydroergotoxine) )Efavirenz Etazepine Etifoxine Fenamates (e.g., flufenamic acid , mefenamic acid , niflumic acid , tolfenamic acid )Fluoxetine Flupirtine Hopantenic acid Lanthanum Lavender oil Lignans (e.g., 4-O-methylhonokiol , honokiol , magnolol , obovatol )Loreclezole Menthyl isovalerate (validolum) Monastrol Niacin Niacinamide Org 25,435 Phenytoin Propanidid Retigabine (ezogabine) Safranal Seproxetine Stiripentol (e.g., sulfonmethane (sulfonal) , tetronal , trional )
Terpenoids (e.g., borneol )Topiramate Valerian constituents (e.g., isovaleric acid , isovaleramide , valerenic acid , )Unsorted benzodiazepine site positive modulators: α-Pinene See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators