Rituximab
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Chimeric (mouse/human) |
| Target | CD20 |
| Clinical data | |
| Trade names | Rituxan, MabThera |
| Biosimilars | rituximab-abbs,[1] rituximab-pvvr,[2] rituximab-arrx[3] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607038 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| Drug class | Monoclonal antibody |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (IV) |
| Elimination half-life | 30 to 400 hours (varies by dose and length of treatment) |
| Excretion | Uncertain: may undergo phagocytosis and catabolism in RES |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.224.382 |
| Chemical and physical data | |
| Formula | C6416H9874N1688O1987S44 |
| Molar mass | 143860.04 g·mol−1 |
| | |
Rituximab, sold under the brand name Rituxan, is a medication used to treat certain autoimmune diseases and types of cancer.[8] It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia(in non-geriatric patients), rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers.[8][9][10][11] It is given by slow injection into a vein.[8] Biosimilars of Rituxan include Blitzima, Riabni, Ritemvia, Rituenza (F.K.A. Tuxella), Rixathon, Ruxience, and Truxima.[12][13]
Common side effects which often occur within two hours of the medication being given include rash, itchiness, low blood pressure, and shortness of breath. Infections are also common.[8]
Severe side effects include reactivation of hepatitis B in those previously infected, progressive multifocal leukoencephalopathy, toxic epidermal necrolysis, and death.[8][14] It is unclear if use during pregnancy is safe for the developing fetus or newborn baby,[4][8] but it is not proven harmful.
Rituximab is a chimeric monoclonal antibody against the protein CD20, which is primarily found on the surface of immune system B cells.[15] When it binds to this protein it triggers cell death.[8]
Rituximab was approved for medical use in 1997.[15] It is on the World Health Organization's List of Essential Medicines.[16]
Medical uses[]
Rituximab is a chimeric monoclonal antibody targeted against CD20 which is a surface antigen present on B cells. Therefore, it acts by depleting normal as well as pathogenic B cells while sparing plasma cells and hematopoietic stem cells as they do not express the CD20 surface antigen.[17]
In the United States, rituximab is indicated to treat:
- Non Hodgkin's Lymphoma (NHL)[7]
- Chronic Lymphocytic Leukemia (CLL)[7]
- Rheumatoid Arthritis having inadequate response to one or more TNF inhibitors[7]
- Vasculitis such as Granulomatosis with polyangiitis and Microscopic polyangiitis[7]
- Moderate to severe Pemphigus vulgaris[7]
Blood cancers[]
Rituximab is used to treat cancers of the white blood system such as leukemias and lymphomas, including non-Hodgkin's lymphoma, chronic lymphocytic leukemia, and lymphocyte predominant subtype, of Hodgkin's Lymphoma.[18] This also includes Waldenström's macroglobulinemia, a type of NHL.[8] Rituximab in combination with hyaluronidase human, sold under the brand names MabThera SC and Rituxan Hycela,[19] is used to treat follicular lymphoma, diffuse large B-cell lymphoma, and chronic lymphocytic leukemia.[19] It is used in combination with fludarabine and cyclophosphamide to treat previously untreated and previously treated CD20-positive chronic lymphocytic leukemia.[7]
Autoimmune diseases[]
Rituximab has been shown to be an effective rheumatoid arthritis treatment in three randomised controlled trials and is now licensed for use in refractory rheumatoid disease.[20] In the United States, it has been FDA-approved for use in combination with methotrexate (MTX) for reducing signs and symptoms in adult patients with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response to one or more anti-TNF-alpha therapy. In the European Union, the license is slightly more restrictive: it is licensed for use in combination with MTX in patients with severe active RA who have had an inadequate response to one or more anti-TNF therapy.[21]
There is some evidence for efficacy, but not necessarily safety, in a range of other autoimmune diseases, and rituximab is widely used off-label to treat difficult cases of multiple sclerosis,[22][23] systemic lupus erythematosus, chronic inflammatory demyelinating polyneuropathy and autoimmune anemias.[24] The most dangerous, although among the most rare, side effect is progressive multifocal leukoencephalopathy (PML) infection, which is usually fatal; however only a very small number of cases have been recorded occurring in autoimmune diseases.[24][25]
Other autoimmune diseases that have been treated with rituximab include autoimmune hemolytic anemia, pure red cell aplasia, thrombotic thrombocytopenic purpura (TTP),[26] idiopathic thrombocytopenic purpura (ITP),[27][28] Evans syndrome,[29] vasculitis (e.g., granulomatosis with polyangiitis), bullous skin disorders (for example pemphigus, pemphigoid—with very encouraging results of approximately 85% rapid recovery in pemphigus, according to a 2006 study),[30] type 1 diabetes mellitus, Sjögren syndrome, anti-NMDA receptor encephalitis and Devic's disease,[31] Graves' ophthalmopathy,[32] autoimmune pancreatitis,[33] Opsoclonus myoclonus syndrome (OMS),[34] and IgG4-related disease.[35] There is some evidence that it is ineffective in treating IgA-mediated autoimmune diseases.[36]
Adverse events[]
Serious adverse events, which can cause death and disability, include:[7][8]
- Severe infusion reaction.
- Cardiac arrest
- Cytokine release syndrome
- Tumor lysis syndrome, causing acute kidney injury
- Infections
- Hepatitis B reactivation
- Other viral infections
- Progressive multifocal leukoencephalopathy (PML) caused by JC virus reactivation[37]
- Immune toxicity, with depletion of B cells in 70% to 80% of lymphoma patients
- Pulmonary toxicity[38]
- Bowel obstruction and perforation[39]
Two patients with systemic lupus erythematosus died of progressive multifocal leukoencephalopathy (PML) after being treated with rituximab. PML is caused by activation of JC virus, a common virus in the brain which is usually latent. Reactivation of the JC virus usually results in death or severe brain damage.[40]
At least one patient with rheumatoid arthritis developed PML after treatment with rituximab.[41]
Rituximab has been reported as a possible cofactor in a chronic Hepatitis E infection in a person with lymphoma. Hepatitis E infection is normally an acute infection, suggesting the drug in combination with lymphoma may have weakened the body's immune response to the virus.[42]
Mechanisms of action[]
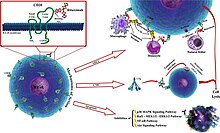

The antibody binds to the cell surface protein CD20. CD20 is widely expressed on B cells, from early pre-B cells to later in differentiation, but it is absent on terminally differentiated plasma cells. Although the function of CD20 is unknown, it may play a role in Ca2+ influx across plasma membranes, maintaining intracellular Ca2+ concentration and allowing activation of B cells.
Rituximab is relatively ineffective in elimination of cells with low CD20 cell-surface levels. It tends to stick to one side of B cells, where CD20 is, forming a cap and drawing proteins over to that side. The presence of the cap changes the effectiveness of natural killer (NK) cells in destroying these B cells. When an NK cell latched onto the cap, it had an 80% success rate at killing the cell. In contrast, when the B cell lacked this asymmetric protein cluster, it was killed only 40% of the time.[44][45]
The following effects have been found:[46]
- The Fc portion of rituximab mediates antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
- Rituximab has a general regulatory effect on the cell cycle.
- Preferential elimination of malignant B cells with high CD20 levels and high BCR signaling propensity, especially in chronic lymphocytic leukemia (CLL).
- It increases MHC II and adhesion molecules LFA-1 and LFA-3 (lymphocyte function-associated antigen).
- It elicits shedding of CD23.
- It downregulates the B cell receptor.
- It induces apoptosis of CD20+ cells.
The combined effect results in the elimination of B cells (including the cancerous ones) from the body, allowing a new population of healthy B cells to develop from lymphoid stem cells.
Rituximab binds to amino acids 170-173 and 182-185 on CD20, which are physically close to each other as a result of a disulfide bond between amino acids 167 and 183.[47]
Rituximab is co-marketed by Biogen and Genentech in the U.S., by Hoffmann-La Roche in Canada and the European Union, Chugai Pharmaceuticals, Zenyaku Kogyo in Japan and AryoGen in Iran.[48]
History[]
Rituximab was developed by researchers Nabil Hanna, Mitchell Reff, and coworkers at IDEC Pharmaceuticals under the name IDEC-C2B8. The U.S. patent for the drug was issued in 1998 and expired in 2015.[49]
Based on its safety and effectiveness in clinical trials,[50] rituximab was approved by the U.S. Food and Drug Administration in 1997 to treat B-cell non-Hodgkin lymphomas resistant to other chemotherapy regimens.[51] Rituximab, in combination with CHOP chemotherapy, is superior to CHOP alone in the treatment of diffuse large B-cell lymphoma and many other B-cell lymphomas.[52] In 2010, it was approved by the European Commission for maintenance treatment after initial treatment of follicular lymphoma.[53]
It is on the World Health Organization's List of Essential Medicines.[16]
In 2014, Genentech reclassified rituxan as a specialty drug, a class of drugs that are only available through specialty distributors in the US.[54] Because wholesalers discounts and rebates no longer apply, hospitals would pay more.[54]
Originally available for intravenous injection (e.g. over 2.5 hrs), in 2016, it gained EU approval in a formulation for subcutaneous injection for B-cell CLL/lymphoma (CLL).[55]
Patents on the drug in expired in Europe in February 2013, and in the US in September 2016.[failed verification][56] By November 2018, several biosimilars had been approved in the US, India, the European Union, Switzerland, Japan and Australia. The US FDA approved Truxima (rituximab-abbs) in 2018, Ruxience (rituximab-pvvr) in 2019 and Riabni (rituximab-arrx) in 2020. The latter is about $3600 per 500 mg, wholesale, list.[57][56][58][59]
Research[]
Chronic fatigue syndrome[]
Rituximab did not improve symptoms in patients with chronic fatigue syndrome in a trial published in 2019.[60][61] 22% of participants had serious events.[60] This potential use was investigated after improvements in chronic fatigue syndrome was seen in two cancer patients treated with rituximab.[62]
Intrathecal[]
For CNS diseases, rituximab could be administered intrathecally and this possibility is under study.[63]
Other anti-CD20 monoclonals[]
The efficacy and success of rituximab has led to some other anti-CD20 monoclonal antibodies being developed:
- ocrelizumab, humanized (90%-95% human) B cell-depleting agent.
- ofatumumab (HuMax-CD20) a fully human B cell-depleting agent.[64]
- Third-generation anti-CD20s such as obinutuzumab have a glycoengineered Fc fragment (Fc)[65] with enhanced binding to Fc gamma receptors, which increase ADCC (antibody-dependent cellular cytotoxicity).[66] This strategy for enhancing a monoclonal antibody's ability to induce ADCC takes advantage of the fact that the displayed Fc glycan controls the antibody's affinity for Fc receptors.[67]
References[]
- ^ Jump up to: a b "Truxima- rituximab-abbs injection, solution". DailyMed. Retrieved 26 March 2021.
- ^ Jump up to: a b "Ruxience- rituximab-pvvr injection, solution". DailyMed. Retrieved 26 March 2021.
- ^ Jump up to: a b "Riabni- rituximab-arrx injection, solution". DailyMed. Retrieved 26 March 2021.
- ^ Jump up to: a b "Rituximab Use During Pregnancy". Drugs.com. 16 December 2019. Retrieved 2 February 2020.
- ^ "MabThera 100 mg Concentrate for Solution for Infusion - Summary of Product Characteristics (SmPC)". (emc). 13 March 2021. Retrieved 26 March 2021.
- ^ "MabThera 1400 mg Solution for Subcutaneous Injection - Summary of Product Characteristics (SmPC)". (emc). 13 March 2021. Retrieved 26 March 2021.
- ^ Jump up to: a b c d e f g h "Rituxan- rituximab injection, solution". DailyMed. 6 November 2019. Retrieved 2 February 2020.
- ^ Jump up to: a b c d e f g h i "Rituximab". The American Society of Health-System Pharmacists. Archived from the original on 27 March 2016. Retrieved 8 December 2016.
- ^ Tandan, Rup; Hehir, Michael K.; Waheed, Waqar; Howard, Diantha B. (August 2017). "Rituximab treatment of myasthenia gravis: A systematic review". Muscle & Nerve. 56 (2): 185–196. doi:10.1002/mus.25597. ISSN 1097-4598. PMID 28164324. S2CID 19504332.
- ^ Singer, O; McCune, WJ (May 2017). "Update on maintenance therapy for granulomatosis with polyangiitis and microscopic polyangiitis". Current Opinion in Rheumatology. 29 (3): 248–253. doi:10.1097/BOR.0000000000000382. PMID 28306595. S2CID 35805200.
- ^ Dojcinov SD, Fend F, Quintanilla-Martinez L (March 2018). "EBV-Positive Lymphoproliferations of B- T- and NK-Cell Derivation in Non-Immunocompromised Hosts". Pathogens (Basel, Switzerland). 7 (1): 28. doi:10.3390/pathogens7010028. PMC 5874754. PMID 29518976.
- ^ "Rituximab Biosimilars Shown to Be Safe and Effective". www.medscape.com. Archived from the original on 15 March 2018. Retrieved 29 November 2017.
- ^ "rituximab | Ligand page | IUPHAR/BPS Guide to PHARMACOLOGY". www.guidetopharmacology.org.
- ^ "Boxed Warning and new recommendations to decrease risk of hepatitis B reactivation with the immune-suppressing and anti-cancer drugs Arzerra (ofatumumab) and Rituxan (rituximab)". U.S. Food and Drug Administration (FDA). 29 February 2016. Retrieved 2 February 2020.
- ^ Jump up to: a b Bosch, Xavier; Ramos-Casals, Manuel; Khamashta, Munther A. (2013). Drugs Targeting B-Cells in Autoimmune Diseases. Springer Science & Business Media. pp. 1–4. ISBN 9783034807067. Archived from the original on 5 November 2017.
- ^ Jump up to: a b World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ De A, Ansari A, Sharma N, Sarda A (2017). "Shifting Focus in the Therapeutics of Immunobullous Disease". Indian J Dermatol. 62 (3): 282–90. doi:10.4103/ijd.IJD_199_17 (inactive 2 August 2021). PMC 5448263. PMID 28584371.CS1 maint: DOI inactive as of August 2021 (link)
- ^ Saini KS, Azim HA Jr, Cocorocchio E, Vanazzi A, Saini ML, Raviele PR, Pruneri G, Peccatori FA (2011). "Rituximab in Hodgkin lymphoma: Is the target always a hit?". Cancer Treat Rev. 37 (5): 385–90. doi:10.1016/j.ctrv.2010.11.005. PMID 21183282.
- ^ Jump up to: a b "Rituxan Hycela- rituximab and hyaluronidase injection, solution". DailyMed. 3 December 2019. Retrieved 2 February 2020.
- ^ Edwards J, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close D, Stevens R, Shaw T (2004). "Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis". N Engl J Med. 350 (25): 2572–81. doi:10.1056/NEJMoa032534. PMID 15201414.
- ^ Tak PP, Kalden JR (2011). "Advances in rheumatology: new targeted therapeutics". Arthritis Research & Therapy. 13 (Suppl 1): S5. doi:10.1186/1478-6354-13-S1-S5. PMC 3123966. PMID 21624184.
- ^ McGinley, MP; Moss, BP; Cohen, JA (January 2017). "Safety of monoclonal antibodies for the treatment of multiple sclerosis". Expert Opinion on Drug Safety. 16 (1): 89–100. doi:10.1080/14740338.2017.1250881. PMID 27756172. S2CID 36762194.
- ^ He, Dian; Guo, Rui; Zhang, Fubo; Zhang, Chao; Dong, Shuai; Zhou, Hongyu (6 December 2013). "Rituximab for relapsing-remitting multiple sclerosis". The Cochrane Database of Systematic Reviews (12): CD009130. doi:10.1002/14651858.CD009130.pub3. ISSN 1469-493X. PMID 24310855.
- ^ Jump up to: a b Paul, Marla (20 May 2009). "Popular Cancer Drug Linked to Often Fatal 'Brain Eating' Virus". Northwestern University News and Information. Archived from the original on 29 May 2010. Retrieved 22 May 2009.
- ^ "FDA Warns of Safety Concern Regarding Rituxan in New Patient Population". U.S. Food and Drug Administration (FDA) (Press release). 18 December 2006. Archived from the original on 13 May 2009. Retrieved 29 April 2013.
- ^ Froissart A, Veyradier A, Hié M, Benhamou Y, Coppo P (August 2015). "Rituximab in autoimmune thrombotic thrombocytopenic purpura: A success story". Eur J Intern Med. 26 (9): 659–65. doi:10.1016/j.ejim.2015.07.021. PMID 26293834.
- ^ Braendstrup P, Bjerrum OW, Nielsen OJ, et al. (April 2005). "Rituximab chimeric anti-CD20 monoclonal antibody treatment for adult refractory idiopathic thrombocytopenic purpura". Am. J. Hematol. 78 (4): 275–80. doi:10.1002/ajh.20276. PMID 15795920. S2CID 42218074.
- ^ Patel V, Mihatov N, Cooper N, Stasi R, Cunningham-Rundles S, Bussel JB (2007). "Long-term responses seen with rituximab in patients with ITP" (PDF). Community Oncology. 4 (2): 107. doi:10.1016/s1548-5315(11)70061-4. Archived from the original (PDF) on 29 September 2007. Retrieved 18 April 2007.
- ^ Shanafelt TD, Madueme HL, Wold RC, Tefferi A (2003). "Rituximab for Immune Cytopenia in Adults: Idiopathic Thrombocytopenic Purpura, Autoimmune Hemolytic Anemia, and Evans Syndrome" (PDF). Mayo Clinic Proceedings. 78 (11): 1340–1346. doi:10.4065/78.11.1340. PMID 14601692. Archived from the original (PDF) on 13 March 2006.
- ^ A. Razzaque Ahmed, M.D.; Zachary Spigelman, M.D.; Lisa A. Cavacini, Ph.D.; Marshall R. Posner, M.D. (26 October 2006). "Treatment of Pemphigus Vulgaris with Rituximab and Intravenous Immune Globulin". N Engl J Med. 355 (17): 1772–1779. doi:10.1056/nejmoa062930. PMID 17065638.
- ^ Jacob A, Weinshenker BG, Violich I, McLinskey N, Krupp L, Fox RJ, Wingerchuk DM, Boggild M, Constantinescu CS, Miller A, De Angelis T, Matiello M, Cree BA (2008). "Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients". Arch Neurol. 65 (11): 1443–1448. doi:10.1001/archneur.65.11.noc80069. PMID 18779415.
- ^ "Rituximab Treatment of Patients with Severe, Corticosteroid-Resistant Thyroid-Associated Ophthalmopathy". Archived from the original on 22 August 2017. Retrieved 19 October 2011.
- ^ Hart (1), Phil A.; Chari (2), Suresh T. (11 June 2013). "Immunomodulators and Rituximab in the Management of Autoimmune Pancreatitis". Pancreapedia: The Exocrine Pancreas Knowledge Base. doi:10.3998/panc.2013.20. Archived from the original on 2 May 2014. Retrieved 30 April 2014.
- ^ Pranzatelli, M. R. (2004). "Immunologic and Clinical Responses to Rituximab in a Child with Opsoclonus-Myoclonus Syndrome". Pediatrics. 115 (1): e115–9. doi:10.1542/peds.2004-0845. PMID 15601813.
- ^ Khosroshahi, A.; Wallace, Z. S.; Crowe, J. L.; Akamizu, T.; Azumi, A.; Carruthers, M. N.; Chari, S. T.; Della-Torre, E.; Frulloni, L.; Goto, H.; Hart, P. A.; Kamisawa, T.; Kawa, S.; Kawano, M.; Kim, M. H.; Kodama, Y.; Kubota, K.; Lerch, M. M.; Löhr, M.; Masaki, Y.; Matsui, S.; Mimori, T.; Nakamura, S.; Nakazawa, T.; Ohara, H.; Okazaki, K.; Ryu, J.H.; Saeki, T.; Schleinitz, N.; et al. (2015). "International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease". Arthritis & Rheumatology. 67 (7): 1688–1699. doi:10.1002/art.39132. PMID 25809420. S2CID 39750214.
- ^ He Y, Shimoda M, Ono Y, Villalobos IB, Mitra A, Konia T, Grando SA, Zone JJ, Maverakis E (2015). "Persistence of Autoreactive IgA-Secreting B Cells Despite Multiple Immunosuppressive Medications Including Rituximab". JAMA Dermatol. 151 (6): 646–50. doi:10.1001/jamadermatol.2015.59. PMID 25901938.
- ^ Molloy, Eamonn S.; Calabrese, Leonard H. (2012). "Progressive multifocal leukoencephalopathy associated with immunosuppressive therapy in rheumatic diseases: Evolving role of biologic therapies". Arthritis & Rheumatism. 64 (9): 3043–3051. doi:10.1002/art.34468. PMID 22422012.
- ^ Burton C, Kaczmarski R, Jan-Mohamed R (2003). "Interstitial pneumonitis related to rituximab therapy". N Engl J Med. 348 (26): 2690–1, discussion 2690–1. doi:10.1056/NEJM200306263482619. PMID 12826649.
- ^ "Reports of Bowel Obstruction and Perforation with RITUXAN (rituximab)" (PDF). Roche Canada. 10 November 2006. Archived from the original (PDF) on 27 March 2014.
- ^ "Rituximab (marketed as Rituxan) Information". U.S. Food and Drug Administration (FDA). 23 July 2015. Archived from the original on 15 November 2009. Retrieved 15 November 2009.
- ^ "Rituximab, RA and PML" (PDF). U.S. Food and Drug Administration (FDA). Archived from the original (PDF) on 16 September 2008. Retrieved 14 September 2008.
- ^ Kriston, Levente (2009). "Challenges in Reporting Meta-analyses of Diagnostic Accuracy Studies". Annals of Internal Medicine. 150 (6): 430. doi:10.7326/0003-4819-150-6-200903170-00025. PMID 19293085.
- ^ Seyfizadeh, Narges; Seyfizadeh, Nayer; Hasenkamp, J; Huerta-Yepez, S (2016). "A molecular perspective on rituximab: A monoclonal antibody for B cell non Hodgkin lymphoma and other affections". Crit Rev Oncol Hematol. 97: 275–290. doi:10.1016/j.critrevonc.2015.09.001. PMID 26443686.
- ^ Rudnicka, D.; Oszmiana, A.; Finch, D. K.; Strickland, I.; Schofield, D. J.; Lowe, D. C.; Sleeman, M. A.; Davis, D. M. (2013). "Scientists discover why a specific cancer drug is so effective". Blood. 121 (23): 4694–4702. doi:10.1182/blood-2013-02-482570. PMID 23613524. Archived from the original on 3 May 2013. Retrieved 29 April 2013.
- ^ Rudnicka, D.; Oszmiana, A.; Finch, D. K.; Strickland, I.; Schofield, D. J.; Lowe, D. C.; Sleeman, M. A.; Davis, D. M. (2013). "Rituximab causes a polarization of B cells that augments its therapeutic function in NK-cell-mediated antibody-dependent cellular cytotoxicity". Blood. 121 (23): 4694–4702. doi:10.1182/blood-2013-02-482570. PMID 23613524.
- ^ Shaw, T. (2003). "B cell therapy for rheumatoid arthritis: The rituximab (anti-CD20) experience". Annals of the Rheumatic Diseases. 62 (90002): 55ii–59. doi:10.1136/ard.62.suppl_2.ii55. PMC 1766758. PMID 14532151.
- ^ Binder M, Otto F, Mertelsmann R, Veelken H, Trepel M. (2006). "The epitope recognized by rituximab". Blood. 108 (6): 1975–1978. doi:10.1182/blood-2006-04-014639. PMID 16705086.CS1 maint: multiple names: authors list (link)
- ^ "FDA Approves Rituxan Plus Chemotherapy for the Most Common Type of Adult Leukemia | Biogen".
- ^ DrugBank DB00073 Archived 2014-01-05 at the Wayback Machine
- ^ Maloney DG, Grillo-López AJ, White CA, et al. (September 1997). "IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma". Blood. 90 (6): 2188–95. doi:10.1182/blood.V90.6.2188. PMID 9310469.
- ^ Scott, Shane D. (1998). "Rituximab: A New Therapeutic Monoclonal Antibody for Non-Hodgkin's Lymphoma". Cancer Practice. 6 (3): 195–197. doi:10.1046/j.1523-5394.1998.006003195.x. PMID 9652253.
- ^ Harrison's Principles of Internal Medicine, Longo et al. McGraw Hill Medical 2011 page 931
- ^ "Roche Gets EC Nod for Follicular Lymphoma Maintenance Therapy". 29 October 2010. Archived from the original on 31 October 2010.
- ^ Jump up to: a b Saporito, Bill (27 October 2014). "Hospitals Furious at Cancer-Drug Price Hikes". Time. Archived from the original on 20 October 2015. Retrieved 26 October 2015.
- ^ "EU approves second indication for subcutaneous form of Roche's rituximab". Archived from the original on 7 June 2016. Retrieved 9 June 2016.
- ^ Jump up to: a b "Biosimilars of Rituximab". Generics and Biosimilars Initiative. 14 April 2017. Archived from the original on 31 March 2017. Retrieved 29 April 2017.
- ^ "FDA approves first biosimilar for treatment of adult patients with non-Hodgkin's lymphoma". U.S. Food and Drug Administration (FDA) (Press release). 28 November 2018. Retrieved 11 November 2019.
- ^ Lahiri, Diptendu; Osterman, Cynthia (2 November 2018). "Novartis abandons effort for U.S. approval of biosimilar rituximab". Reuters. Retrieved 3 November 2018.
- ^ "Amgen Rituximab Biosimilar Gains FDA Approval". The Center For Biosimilars.
- ^ Jump up to: a b Fluge, Øystein; Rekeland, Ingrid G.; Lien, Katarina; Thürmer, Hanne; Borchgrevink, Petter C.; Schäfer, Christoph; Sørland, Kari; Aßmus, Jörg; Ktoridou-Valen, Irini (2 April 2019). "B-Lymphocyte Depletion in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial". Annals of Internal Medicine. 170 (9): 585–593. doi:10.7326/M18-1451. ISSN 0003-4819. PMID 30934066. S2CID 91186383.
- ^ Tucker, Miriam E. (2 April 2019). "Rituximab Fails to Improve Symptoms in ME/CFS". Medscape.
- ^ Castro-Marrero, Jesus; Sáez-Francàs, Naia; Santillo, Dafna; Alegre, Jose (2017). "Treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis: All roads lead to Rome". British Journal of Pharmacology. 174 (5): 345–369. doi:10.1111/bph.13702. PMC 5301046. PMID 28052319.
- ^ Bonnan, M; Ferrari, S; Bertandeau, E; Demasles, S; Krim, E; Miquel, M; Barroso, B (2014). "Intrathecal rituximab therapy in multiple sclerosis: review of evidence supporting the need for future trials". Current Drug Targets. 15 (13): 1205–14. doi:10.2174/1389450115666141029234644. PMID 25355180.
- ^ "Genmab.com / HuMax-CD20 (ofatumumab)". Archived from the original on 11 September 2007. Retrieved 3 December 2007.
- ^ "Fc-structure". Archived from the original on 10 November 2007. Retrieved 3 December 2007.
- ^ Eccles, SA (2001). "Monoclonal antibodies targeting cancer: 'magic bullets' or just the trigger?". Breast Cancer Research. 3 (2): 86–90. doi:10.1186/bcr276. PMC 138676. PMID 11250751.
- ^ Maverakis E, Kim K, Shimoda M, Gershwin M, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB (2015). "Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: A critical review". J Autoimmun. 57 (6): 1–13. doi:10.1016/j.jaut.2014.12.002. PMC 4340844. PMID 25578468.
External links[]
- "Rituximab". Drug Information Portal. U.S. National Library of Medicine.
- "Hyaluronidase mixture with Rituximab". Drug Information Portal. U.S. National Library of Medicine.
- "Rituximab and Hyaluronidase (Professional Patient Advice)". Drugs.com.
- "Rituximab and Hyaluronidase Human Injection". MedlinePlus.
- Genentech brands
- Hoffmann-La Roche brands
- Monoclonal antibodies for tumors
- Orphan drugs
- Specialty drugs
- World Health Organization essential medicines